Comparative safety and effectiveness of serotonin receptor antagonists in patients undergoing chemotherapy: a systematic review and network meta-analysis
- PMID: 28007031
- PMCID: PMC5180412
- DOI: 10.1186/s12916-016-0761-9
Comparative safety and effectiveness of serotonin receptor antagonists in patients undergoing chemotherapy: a systematic review and network meta-analysis
Abstract
Background: Although serotonin (5-HT3) receptor antagonists are effective in reducing nausea and vomiting, they may be associated with increased cardiac risk. Our objective was to examine the comparative safety and effectiveness of 5-HT3 receptor antagonists (e.g., dolasetron, granisetron, ondansetron, palonosetron, tropisetron) alone or combined with steroids for patients undergoing chemotherapy.
Methods: We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception until December 2015 for studies comparing 5-HT3 receptor antagonists with each other or placebo in chemotherapy patients. The search results were screened, data were abstracted, and risk of bias was appraised by pairs of reviewers, independently. Random-effects meta-analyses and network meta-analyses (NMAs) were conducted.
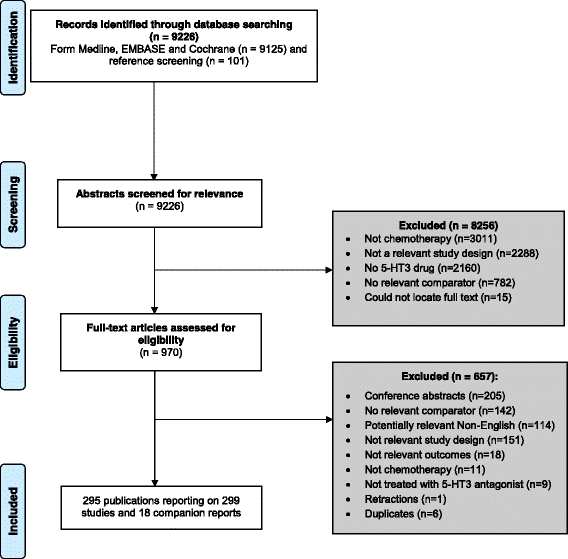
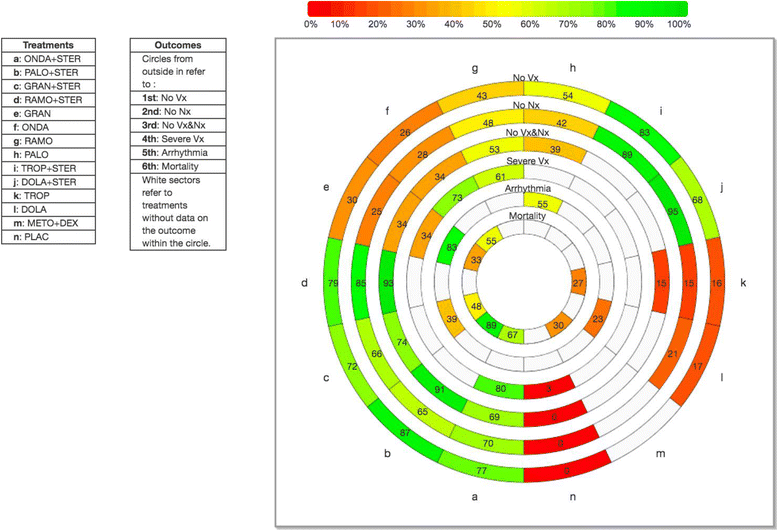
Results: After screening 9226 citations and 970 full-text articles, we included 299 studies (n = 58,412 patients). None of the included studies reported harms for active treatment versus placebo. For NMAs on the risk of arrhythmia (primary outcome; three randomized controlled trials [RCTs], 627 adults) and mortality (secondary outcome; eight RCTs, 4823 adults), no statistically significant differences were observed between agents. A NMA on the risk of QTc prolongation showed a significantly greater risk for dolasetron + dexamethasone versus ondansetron + dexamethasone (four RCTs, 3358 children and adults, odds ratio 2.94, 95% confidence interval 2.13-4.17). For NMAs on the number of patients without nausea (44 RCTs, 11,664 adults, 12 treatments), number of patients without vomiting (63 RCTs, 15,460 adults, 12 treatments), and number of patients without chemotherapy-induced nausea or vomiting (27 RCTs, 10,924 adults, nine treatments), all agents were significantly superior to placebo. For a NMA on severe vomiting (10 RCTs, 917 adults), all treatments decreased the risk, but only ondansetron and ramosetron were significantly superior to placebo. According to a rank-heat plot with the surface under the cumulative ranking curve results, palonosetron + steroid was ranked the safest and most effective agent overall.
Conclusions: Most 5-HT3 receptor antagonists were relatively safe when compared with each other, yet none of the studies compared active treatment with placebo for harms. However, dolasetron + dexamethasone may prolong the QTc compared to ondansetron + dexamethasone. All agents were effective for reducing risk of nausea, vomiting, and chemotherapy-induced nausea or vomiting.
Trial registration: This study was registered at PROSPERO: ( CRD42013003564 ).
Keywords: Chemotherapy; Effectiveness; Network meta-analysis; Safety; Serotonin receptor antagonists; Systematic review.
Figures

Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood.Cochrane Database Syst Rev. 2016 Feb 2;2(2):CD007786. doi: 10.1002/14651858.CD007786.pub3. Cochrane Database Syst Rev. 2016. PMID: 26836199 Free PMC article.
-
Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults.Cochrane Database Syst Rev. 2018 Sep 21;9(9):CD012555. doi: 10.1002/14651858.CD012555.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246876 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Apatinib-loaded lipid nanobubbles combined with ultrasound-targeted nanobubble destruction for synergistic treatment of HepG2 cells in vitro.Onco Targets Ther. 2018 Aug 10;11:4785-4795. doi: 10.2147/OTT.S170786. eCollection 2018. Onco Targets Ther. 2018. PMID: 30127626 Free PMC article.
-
Research trends on chemotherapy induced nausea and vomiting: a bibliometric analysis.Front Pharmacol. 2024 Sep 13;15:1369442. doi: 10.3389/fphar.2024.1369442. eCollection 2024. Front Pharmacol. 2024. PMID: 39346558 Free PMC article.
-
Postoperative Nausea and Vomiting in Paediatric Anaesthesia.Turk J Anaesthesiol Reanim. 2020 Apr;48(2):88-95. doi: 10.5152/TJAR.2019.67503. Epub 2019 Nov 11. Turk J Anaesthesiol Reanim. 2020. PMID: 32259138 Free PMC article. Review.
-
Adequacy of recommendations for adverse event management in national and international treatment guidelines for rifampicin-susceptible tuberculosis: a systematic review.EClinicalMedicine. 2025 Mar 18;82:103148. doi: 10.1016/j.eclinm.2025.103148. eCollection 2025 Apr. EClinicalMedicine. 2025. PMID: 40166655 Free PMC article. Review.
-
Efficacy of olanzapine, neurokinin-1 receptor antagonists, and thalidomide in combination with palonosetron plus dexamethasone in preventing highly emetogenic chemotherapy-induced nausea and vomiting: a Bayesian network meta-analysis.Support Care Cancer. 2020 Mar;28(3):1031-1039. doi: 10.1007/s00520-019-05210-4. Epub 2019 Dec 10. Support Care Cancer. 2020. PMID: 31823054
References
-
- Berger AM, Clark-Snow RA. Nausea and vomiting. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice in oncology. 6. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2869–2880.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

