RGMs: Structural Insights, Molecular Regulation, and Downstream Signaling
- PMID: 28007423
- PMCID: PMC5404723
- DOI: 10.1016/j.tcb.2016.11.009
RGMs: Structural Insights, Molecular Regulation, and Downstream Signaling
Abstract
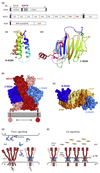
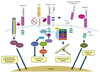
Although originally discovered as neuronal growth cone-collapsing factors, repulsive guidance molecules (RGMs) are now known as key players in many fundamental processes, such as cell migration, differentiation, iron homeostasis, and apoptosis, during the development and homeostasis of many tissues and organs, including the nervous, skeletal, and immune systems. Furthermore, three RGMs (RGMa, RGMb/DRAGON, and RGMc/hemojuvelin) have been linked to the pathogenesis of various disorders ranging from multiple sclerosis (MS) to cancer and juvenile hemochromatosis (JHH). While the molecular details of these (patho)biological effects and signaling modes have long remained unknown, recent studies unveil several exciting and novel aspects of RGM processing, ligand-receptor interactions, and downstream signaling. In this review, we highlight recent advances in the mechanisms-of-action and function of RGM proteins.
Keywords: BMP; axon guidance; immune system; iron metabolism; neogenin; proteolytic cleavage.
Crown Copyright © 2016. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Kolodkin AL, Pasterkamp RJ. SnapShot: axon guidance II. Cell. 2013;153:722.e1. - PubMed
-
- Rajagopalan S, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. - PubMed
-
- Matsunaga E, et al. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–755. - PubMed
-
- Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

