Paternal Insulin-like Growth Factor 2 (Igf2) Regulates Stem Cell Activity During Adulthood
- PMID: 28007480
- PMCID: PMC5233811
- DOI: 10.1016/j.ebiom.2016.11.035
Paternal Insulin-like Growth Factor 2 (Igf2) Regulates Stem Cell Activity During Adulthood
Abstract
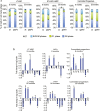
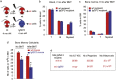
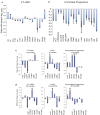
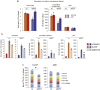
Insulin-like Growth Factor 2 (IGF2) belongs to the IGF/Insulin pathway, a highly conserved evolutionarily network that regulates growth, aging and lifespan. Igf2 is highly expressed in the embryo and in cancer cells. During mouse development, Igf2 is expressed in all sites where hematopoietic stem cells (HSC) successively expand, then its expression drops at weaning and becomes undetectable when adult HSC have reached their niches in bones and start to self-renew. In the present study, we aim to discover the role of IGF2 during adulthood. We show that Igf2 is specifically expressed in adult HSC and we analyze HSC from adult mice deficient in Igf2 transcripts. We demonstrate that Igf2 deficiency avoids the age-related attrition of the HSC pool and that Igf2 is necessary for tissue homeostasis and regeneration. Our study reveals that the expression level of Igf2 is critical to maintain the balance between stem cell self-renewal and differentiation, presumably by regulating the interaction between HSC and their niche. Our data have major clinical interest for transplantation: understanding the changes in adult stem cells and their environments will improve the efficacy of regenerative medicine and impact health- and life-span.
Keywords: Adulthood; Igf2; Imprinting; Longevity; Niche; Self-renewal; Stem cells.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Alvarez-Silva M., Belo-Diabangouaya P., Salaün J., Dieterlin-Lièvre F. Mouse placenta is a major hematopoietic organ. Development. 2003;130:5437–5444. - PubMed
-
- Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
-
- Bartolomei M.S., Zemel S., Tilghman S.M. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–155. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

