Control of Ventilation in Health and Disease
- PMID: 28007622
- PMCID: PMC8894312
- DOI: 10.1016/j.chest.2016.12.002
Control of Ventilation in Health and Disease
Abstract
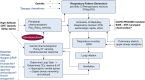
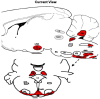
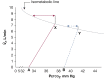
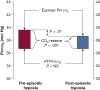
Control of ventilation occurs at different levels of the respiratory system through a negative feedback system that allows precise regulation of levels of arterial carbon dioxide and oxygen. Mechanisms for ventilatory instability leading to sleep-disordered breathing include changes in the genesis of respiratory rhythm and chemoresponsiveness to hypoxia and hypercapnia, cerebrovascular reactivity, abnormal chest wall and airway reflexes, and sleep state oscillations. One can potentially stabilize breathing during sleep and treat sleep-disordered breathing by identifying one or more of these pathophysiological mechanisms. This review describes the current concepts in ventilatory control that pertain to breathing instability during wakefulness and sleep, delineates potential avenues for alternative therapies to stabilize breathing during sleep, and proposes recommendations for future research.
Published by Elsevier Inc.
Figures

References
-
- Richter D.W., Spyer K.M. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24(8):464–472. - PubMed
-
- Feldman J., Janczewski W. Point: Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Counterpoint: the pre-BötC is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol (1985) 2006;100(6):2096. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

