H255Y and K508R missense mutations in tumour suppressor folliculin (FLCN) promote kidney cell proliferation
- PMID: 28007907
- PMCID: PMC6075457
- DOI: 10.1093/hmg/ddw392
H255Y and K508R missense mutations in tumour suppressor folliculin (FLCN) promote kidney cell proliferation
Abstract
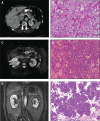
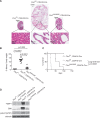
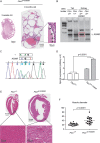
Germline H255Y and K508R missense mutations in the folliculin (FLCN) gene have been identified in patients with bilateral multifocal (BMF) kidney tumours and clinical manifestations of Birt-Hogg-Dubé (BHD) syndrome, or with BMF kidney tumours as the only manifestation; however, their impact on FLCN function remains to be determined. In order to determine if FLCN H255Y and K508R missense mutations promote aberrant kidney cell proliferation leading to pathogenicity, we generated mouse models expressing these mutants using BAC recombineering technology and investigated their ability to rescue the multi-cystic phenotype of Flcn-deficient mouse kidneys. Flcn H255Y mutant transgene expression in kidney-targeted Flcn knockout mice did not rescue the multi-cystic kidney phenotype. However, expression of the Flcn K508R mutant transgene partially, but not completely, abrogated the phenotype. Notably, expression of the Flcn K508R mutant transgene in heterozygous Flcn knockout mice resulted in development of multi-cystic kidneys and cardiac hypertrophy in some mice. These results demonstrate that both FLCN H255Y and K508R missense mutations promote aberrant kidney cell proliferation, but to different degrees. Based on the phenotypes of our preclinical models, the FLCN H255Y mutant protein has lost it tumour suppressive function leading to the clinical manifestations of BHD, whereas the FLCN K508R mutant protein may have a dominant negative effect on the function of wild-type FLCN in regulating kidney cell proliferation and, therefore, act as an oncoprotein. These findings may provide mechanistic insight into the role of FLCN in regulating kidney cell proliferation and facilitate the development of novel therapeutics for FLCN-deficient kidney cancer.
Published by Oxford University Press 2016. This work is written by US Government employees and is in the public domain in the US.
Figures



References
-
- Birt A. R., Hogg G. R., Dube W. J. (1977) Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch. Dermatol., 113, 1674–1677. - PubMed
-
- Zbar B., Alvord W.G., Glenn G., Turner M., Pavlovich C.P., Schmidt L., McClellan W., Choyke P., Weirich G., Hewitt S. M., et al. (2002) Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol. Biomarkers Prev ., 11, 393–400. - PubMed
-
- Nickerson M. L., Warren M.B., Toro J.R., Matrosova V., Glenn G., Turner M.L., Duray P., Merino M., Choyke P., Pavlovich C.P., et al. (2002) Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell, 2, 157–164. - PubMed
-
- Baba M., Hong S.B., Sharma N., Warren M.B., Nickerson M.L., Iwamatsu A., Esposito D., Gillette W.K., Hopkins R.F. III, Hartley J.L., et al. (2006) Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl. Acad. Sci. USA, 103, 15552–15557. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

