Vascular Endothelial Growth Factor (VEGF) Promotes Assembly of the p130Cas Interactome to Drive Endothelial Chemotactic Signaling and Angiogenesis
- PMID: 28007913
- PMCID: PMC5294206
- DOI: 10.1074/mcp.M116.064428
Vascular Endothelial Growth Factor (VEGF) Promotes Assembly of the p130Cas Interactome to Drive Endothelial Chemotactic Signaling and Angiogenesis
Abstract
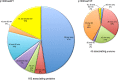
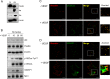
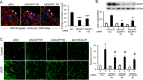
p130Cas is a polyvalent adapter protein essential for cardiovascular development, and with a key role in cell movement. In order to identify the pathways by which p130Cas exerts its biological functions in endothelial cells we mapped the p130Cas interactome and its dynamic changes in response to VEGF using high-resolution mass spectrometry and reconstruction of protein interaction (PPI) networks with the aid of multiple PPI databases. VEGF enriched the p130Cas interactome in proteins involved in actin cytoskeletal dynamics and cell movement, including actin-binding proteins, small GTPases and regulators or binders of GTPases. Detailed studies showed that p130Cas association of the GTPase-binding scaffold protein, IQGAP1, plays a key role in VEGF chemotactic signaling, endothelial polarization, VEGF-induced cell migration, and endothelial tube formation. These findings indicate a cardinal role for assembly of the p130Cas interactome in mediating the cell migratory response to VEGF in angiogenesis, and provide a basis for further studies of p130Cas in cell movement.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 - PubMed
-
- Ferrara N., Gerber H. P., and LeCouter J. (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676 - PubMed
-
- Neufeld G., Cohen T., Gengrinovitch S., and Poltorak Z. (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9–22 - PubMed
-
- Zachary I. (2003) VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem. Soc. Trans. 31, 1171–1177 - PubMed
-
- Dougher M., and Terman B. I. (1999) Autophosphorylation of VEGFR2 in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 18, 1619–1627 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

