c-JUN Dimerization Protein 2 (JDP2) Is a Transcriptional Repressor of Follicle-stimulating Hormone β (FSHβ) and Is Required for Preventing Premature Reproductive Senescence in Female Mice
- PMID: 28007961
- PMCID: PMC5314163
- DOI: 10.1074/jbc.M116.771808
c-JUN Dimerization Protein 2 (JDP2) Is a Transcriptional Repressor of Follicle-stimulating Hormone β (FSHβ) and Is Required for Preventing Premature Reproductive Senescence in Female Mice
Abstract
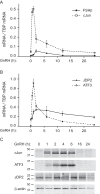
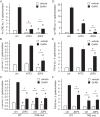
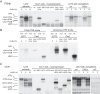
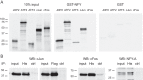
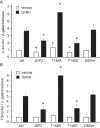
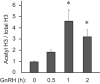
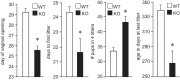
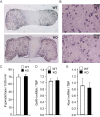
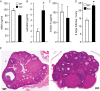
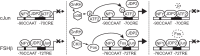
Follicle-stimulating hormone (FSH) regulates follicular growth and stimulates estrogen synthesis in the ovaries. FSH is a heterodimer consisting of an α subunit, also present in luteinizing hormone, and a unique β subunit, which is transcriptionally regulated by gonadotropin-releasing hormone 1 (GNRH). Because most FSH is constitutively secreted, tight transcriptional regulation is critical for maintaining FSH levels within a narrow physiological range. Previously, we reported that GNRH induces FSHβ (Fshb) transcription via induction of the AP-1 transcription factor, a heterodimer of c-FOS and c-JUN. Herein, we identify c-JUN-dimerization protein 2 (JDP2) as a novel repressor of GNRH-mediated Fshb induction. JDP2 exhibited high basal expression and bound the Fshb promoter at an AP-1-binding site in a complex with c-JUN. GNRH treatment induced c-FOS to replace JDP2 as a c-JUN binding partner, forming transcriptionally active AP-1. Subsequently, rapid c-FOS degradation enabled reformation of the JDP2 complex. In vivo studies revealed that JDP2 null male mice have normal reproductive function, as expected from a negative regulator of the FSH hormone. Female JDP2 null mice, however, exhibited early puberty, observed as early vaginal opening, larger litters, and early reproductive senescence. JDP2 null females had increased levels of circulating FSH and higher expression of the Fshb subunit in the pituitary, resulting in elevated serum estrogen and higher numbers of large ovarian follicles. Disruption of JDP2 function therefore appears to cause early cessation of reproductive function, a condition that has been associated with elevated FSH in women.
Keywords: AP-1 transcription factor (AP-1); c-FOS; c-JUN transcription factor; endocrinology; follicle-stimulating hormone (FSH); gene expression; gene transcription.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Ortolano G. A., Haisenleder D. J., Dalkin A. C., Iliff-Sizemore S. A., Landefeld T. D., Maurer R. A., and Marshall J. C. (1988) Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology 123, 2946–2948 - PubMed
-
- Zmeili S. M., Papavasiliou S. S., Thorner M. O., Evans W. S., Marshall J. C., and Landefeld T. D. (1986) α and luteinizing hormone β subunit messenger ribonucleic acids during the rat estrous cycle. Endocrinology 119, 1867–1869 - PubMed
-
- Huhtaniemi I. (2006) Mutations along the pituitary-gonadal axis affecting sexual maturation: novel information from transgenic and knockout mice. Mol. Cell Endocrinol. 254, 84–90 - PubMed
-
- Huhtaniemi I., Ahtiainen P., Pakarainen T., Rulli S. B., Zhang F. P., and Poutanen M. (2006) Genetically modified mouse models in studies of luteinising hormone action. Mol. Cell Endocrinol. 252, 126–135 - PubMed
-
- Lamminen T., Jokinen P., Jiang M., Pakarinen P., Simonsen H., and Huhtaniemi I. (2005) Human FSH beta subunit gene is highly conserved. Mol. Hum. Reprod. 11, 601–605 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

