Phospholipase A2-activating protein is associated with a novel form of leukoencephalopathy
- PMID: 28007986
- PMCID: PMC5841054
- DOI: 10.1093/brain/aww295
Phospholipase A2-activating protein is associated with a novel form of leukoencephalopathy
Abstract
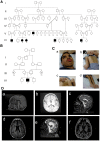
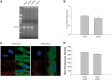
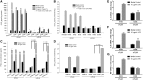
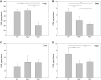
Leukoencephalopathies are a group of white matter disorders related to abnormal formation, maintenance, and turnover of myelin in the central nervous system. These disorders of the brain are categorized according to neuroradiological and pathophysiological criteria. Herein, we have identified a unique form of leukoencephalopathy in seven patients presenting at ages 2 to 4 months with progressive microcephaly, spastic quadriparesis, and global developmental delay. Clinical, metabolic, and imaging characterization of seven patients followed by homozygosity mapping and linkage analysis were performed. Next generation sequencing, bioinformatics, and segregation analyses followed, to determine a loss of function sequence variation in the phospholipase A2-activating protein encoding gene (PLAA). Expression and functional studies of the encoded protein were performed and included measurement of prostaglandin E2 and cytosolic phospholipase A2 activity in membrane fractions of fibroblasts derived from patients and healthy controls. Plaa-null mice were generated and prostaglandin E2 levels were measured in different tissues. The novel phenotype of our patients segregated with a homozygous loss-of-function sequence variant, causing the substitution of leucine at position 752 to phenylalanine, in PLAA, which causes disruption of the protein's ability to induce prostaglandin E2 and cytosolic phospholipase A2 synthesis in patients' fibroblasts. Plaa-null mice were perinatal lethal with reduced brain levels of prostaglandin E2 The non-functional phospholipase A2-activating protein and the associated neurological phenotype, reported herein for the first time, join other complex phospholipid defects that cause leukoencephalopathies in humans, emphasizing the importance of this axis in white matter development and maintenance.
Keywords: autosomal recessive; complex phospholipid defects; phospholipase A2-activating protein (PLAA); progressive leukoencephalopathy; startle response.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Akella A, Deshpande SB. Pulmonary surfactants and their role in pathophysiology of lung disorders. Indian J Exp Biol 2013; 51: 5–22. - PubMed
-
- Atrouni S, Daraze A, Tamraz J, Cassia A, Caillaud C, Megarbane A. Leukodystrophy associated with oligodontia in a large inbred family: fortuitous association or new entity? Am J Med Genet A 2003; 118A: 76–81. - PubMed
-
- Bazan NG, Colangelo V, Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat 2002; 68–9: 197–210. - PubMed
-
- Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet 2000; 26: 370–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

