Rescuing cholinergic neurons from apoptotic degeneration by targeting of serotonin modulator-and apolipoprotein E-conjugated liposomes to the hippocampus
- PMID: 28008255
- PMCID: PMC5170675
- DOI: 10.2147/IJN.S123442
Rescuing cholinergic neurons from apoptotic degeneration by targeting of serotonin modulator-and apolipoprotein E-conjugated liposomes to the hippocampus
Abstract
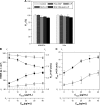
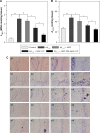
β-Amyloid (Aβ)-targeting liposomes (LIP) with surface serotonin modulator (SM) and apolipoprotein E (ApoE) were utilized to facilitate the delivery of nerve growth factor (NGF) across the blood-brain barrier (BBB) for neuroprotection in the hippocampus. The therapeutic efficacy of SM- and ApoE-grafted LIP carrying NGF (NGF-SM-ApoE-LIP) was assessed by an in vitro Alzheimer's disease (AD) model of degenerated SK-N-MC cells and an in vivo AD model of Aβ-insulted Wistar rats. The experimental evidences revealed that the modified SM and ApoE on the surface of LIP increased the permeation of NGF across the BBB without serious damage to structural integrity of tight junction. When compared with free NGF, NGF-SM-ApoE-LIP upregulated the expression of phosphorylated neurotrophic tyrosine kinase receptor type 1 on cholinergic neurons and significantly improved their survival. In addition, NGF-SM-ApoE-LIP could reduce the secretion of acetylcholinesterase and malondialdehyde and rescue hippocampal neurons from apoptosis in rat brains. The synergistic effect of SM and ApoE is promising in the induction of NGF to inhibit the neurotoxicity of Aβ and NGF-SM-ApoE-LIP can be a potent antiapoptotic pharmacotherapy for clinical care of patients with AD.
Keywords: Alzheimer’s disease; apolipoprotein E; blood–brain barrier; liposome; nerve growth factor; serotonin modulator.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Optimized liposomes with transactivator of transcription peptide and anti-apoptotic drugs to target hippocampal neurons and prevent tau-hyperphosphorylated neurodegeneration.Acta Biomater. 2019 Mar 15;87:207-222. doi: 10.1016/j.actbio.2019.01.065. Epub 2019 Feb 1. Acta Biomater. 2019. PMID: 30716553
-
Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer's disease treatment.Int J Nanomedicine. 2017 Mar 2;12:1757-1774. doi: 10.2147/IJN.S128396. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28280340 Free PMC article.
-
Targeting delivery of liposomes with conjugated p-aminophenyl-α-d-manno-pyranoside and apolipoprotein E for inhibiting neuronal degeneration insulted with β-amyloid peptide.J Drug Target. 2015 Feb;23(2):147-58. doi: 10.3109/1061186X.2014.965716. Epub 2014 Sep 30. J Drug Target. 2015. PMID: 25268274
-
The Intersection of NGF/TrkA Signaling and Amyloid Precursor Protein Processing in Alzheimer's Disease Neuropathology.Int J Mol Sci. 2017 Jun 20;18(6):1319. doi: 10.3390/ijms18061319. Int J Mol Sci. 2017. PMID: 28632177 Free PMC article. Review.
-
Apolipoprotein E, amyloid-beta, and blood-brain barrier permeability in Alzheimer disease.J Neuropathol Exp Neurol. 2008 Apr;67(4):261-70. doi: 10.1097/NEN.0b013e31816a0dc8. J Neuropathol Exp Neurol. 2008. PMID: 18379441 Review.
Cited by
-
Lipid-Based Drug Delivery Systems in Regenerative Medicine.Materials (Basel). 2021 Sep 17;14(18):5371. doi: 10.3390/ma14185371. Materials (Basel). 2021. PMID: 34576594 Free PMC article. Review.
-
Crossing the blood-brain barrier: nanoparticle-based strategies for neurodegenerative disease therapy.Drug Deliv Transl Res. 2025 Jun 14. doi: 10.1007/s13346-025-01887-9. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40517187
-
Cellular Reprogramming and Its Potential Application in Alzheimer's Disease.Front Neurosci. 2022 Apr 7;16:884667. doi: 10.3389/fnins.2022.884667. eCollection 2022. Front Neurosci. 2022. PMID: 35464309 Free PMC article. Review.
-
Liposome based drug delivery as a potential treatment option for Alzheimer's disease.Neural Regen Res. 2022 Jun;17(6):1190-1198. doi: 10.4103/1673-5374.327328. Neural Regen Res. 2022. PMID: 34782553 Free PMC article. Review.
-
The use of nanoparticles in the treatment of infectious diseases and cancer, dental applications and tissue regeneration: a review.Front Med Technol. 2024 Jan 23;5:1330007. doi: 10.3389/fmedt.2023.1330007. eCollection 2023. Front Med Technol. 2024. PMID: 38323112 Free PMC article. Review.
References
-
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. - PubMed
-
- Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:1–79. - PubMed
-
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. - PubMed
-
- Bottger D, Ullrich C, Humpel C. Monocytes deliver bioactive nerve growth factor through a brain capillary endothelial cell-monolayer in vitro and counteract degeneration of cholinergic neurons. Brain Res. 2010;1312:108–119. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

