Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells
- PMID: 28008271
- PMCID: PMC5167467
- DOI: 10.2147/OTT.S115268
Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells
Abstract
Background: Colorectal cancer (CRC) is the third most prevalent cancer worldwide. New prognostic markers are needed to identify patients with poorer prognosis, and circulating tumor cells (CTCs) seem to be promising to accomplish this.
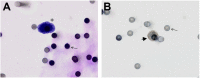
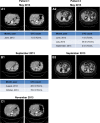
Patients and methods: A prospective study was conducted by blood collection from patients with metastatic CRC (mCRC), three times, every 2 months in conjunction with image examinations for evaluation of therapeutic response. CTC isolation and counting were performed by Isolation by Size of Epithelial Tumor Cells (ISET).
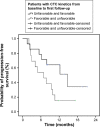
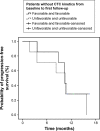
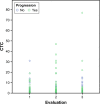
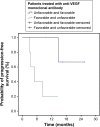
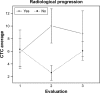
Results: A total of 54 patients with mCRC with a mean age of 57.3 years (31-82 years) were included. Among all patients, 60% (n=32) were carriers of wild-type KRAS (WT KRAS) tumors and 90% of them (n=29) were exposed to monoclonal antibodies along with systemic treatment. Evaluating CTC kinetics, when we compared the baseline (pretreatment) CTC level (CTC1) with the level at first follow-up (CTC2), we observed that CTC1-positive patients (CTCs above the median), who became negative (CTCs below the median) had a favorable evolution (n=14), with a median progression-free survival (PFS) of 14.7 months. This was higher than that for patients with an unfavorable evolution (CTC1- that became CTC2+; n=13, 6.9 months; P=0.06). Patients with WT KRAS with favorable kinetics had higher PFS (14.7 months) in comparison to those with WT KRAS with unfavorable kinetics (9.4 months; P=0.02). Moreover, patients whose imaging studies showed radiological progression had an increased quantification of CTCs at CTC2 compared to those without progression (P=0.04).
Conclusion: This study made possible the presentation of ISET as a feasible tool for evaluating CTC kinetics in patients with mCRC, which can be promising in their clinical evaluation.
Keywords: ISET; circulating tumor cells; kinetics; metastatic colorectal cancer.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013.
-
- Lee WS, Yun SH, Chun HK, et al. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis. 2007;22(6):699–704. - PubMed
-
- Van Cutsem E, Nordlinger B, Adam R, et al. European Colorectal Metastases Treatment Group Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–2221. - PubMed
-
- Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6(3):202–207. - PubMed
-
- Chang GJ. Challenge of primary tumor management in patients with stage IV colorectal cancer. J Clin Oncol. 2012;30:3165–3166. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

