The Protective Role of microRNA-200c in Alzheimer's Disease Pathologies Is Induced by Beta Amyloid-Triggered Endoplasmic Reticulum Stress
- PMID: 28008308
- PMCID: PMC5143617
- DOI: 10.3389/fnmol.2016.00140
The Protective Role of microRNA-200c in Alzheimer's Disease Pathologies Is Induced by Beta Amyloid-Triggered Endoplasmic Reticulum Stress
Abstract
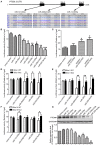
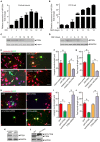
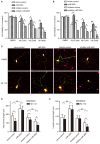
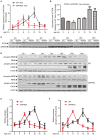
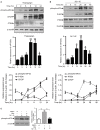
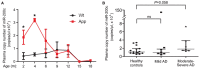
MicroRNAs are small non-coding RNAs that repress the expression of their target proteins. The roles of microRNAs in the development of Alzheimer's disease (AD) are not clear. In this study we show that miR-200c represses the expression of PTEN protein. PTEN downregulation by miR-200c supports the survival and differentiation of cultured neurons. AD is a progressive neurodegenerative disease signified by beta amyloid (Aβ) peptide aggregation and deposition. In a mouse model of AD that is induced by APPswe and PS1ΔE9 double transgenes, we found Aβ deposition results in neuronal ER stress that induces miR200c. Pharmacological blockade of ER stress inhibited Aβ-induced miR-200c overexpression in AD brains. MiR-200c was detected in the serum of both AD mice and human AD patients. These findings suggest that miR-200c functions as part of the neuronal cell-intrinsic adaptive machinery, and supports neuronal survival and differentiation in response to Aβ induced ER-stress by downregulating PTEN.
Keywords: Alzheimer's disease (AD); PTEN; beta amyloid peptide (Aβ); endoplasmic reticulum stress (ER stress); miR-200c; microRNA.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

