Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique?
- PMID: 28008325
- PMCID: PMC5143676
- DOI: 10.3389/fmicb.2016.01936
Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique?
Abstract
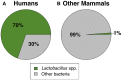
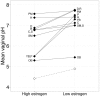
The human vaginal microbiome is dominated by bacteria from the genus Lactobacillus, which create an acidic environment thought to protect women against sexually transmitted pathogens and opportunistic infections. Strikingly, lactobacilli dominance appears to be unique to humans; while the relative abundance of lactobacilli in the human vagina is typically >70%, in other mammals lactobacilli rarely comprise more than 1% of vaginal microbiota. Several hypotheses have been proposed to explain humans' unique vaginal microbiota, including humans' distinct reproductive physiology, high risk of STDs, and high risk of microbial complications linked to pregnancy and birth. Here, we test these hypotheses using comparative data on vaginal pH and the relative abundance of lactobacilli in 26 mammalian species and 50 studies (N = 21 mammals for pH and 14 mammals for lactobacilli relative abundance). We found that non-human mammals, like humans, exhibit the lowest vaginal pH during the period of highest estrogen. However, the vaginal pH of non-human mammals is never as low as is typical for humans (median vaginal pH in humans = 4.5; range of pH across all 21 non-human mammals = 5.4-7.8). Contrary to disease and obstetric risk hypotheses, we found no significant relationship between vaginal pH or lactobacilli relative abundance and multiple metrics of STD or birth injury risk (P-values ranged from 0.13 to 0.99). Given the lack of evidence for these hypotheses, we discuss two alternative explanations: the common function hypothesis and a novel hypothesis related to the diet of agricultural humans. Specifically, with regard to diet we propose that high levels of starch in human diets have led to increased levels of glycogen in the vaginal tract, which, in turn, promotes the proliferation of lactobacilli. If true, human diet may have paved the way for a novel, protective microbiome in human vaginal tracts. Overall, our results highlight the need for continuing research on non-human vaginal microbial communities and the importance of investigating both the physiological mechanisms and the broad evolutionary processes underlying human lactobacilli dominance.
Keywords: estrogen; evolution; lactobacilli; mammals; pH; vaginal microbiome.
Figures


Comment in
-
Commentary: Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique?Front Microbiol. 2017 Sep 25;8:1815. doi: 10.3389/fmicb.2017.01815. eCollection 2017. Front Microbiol. 2017. PMID: 28993762 Free PMC article. No abstract available.
References
-
- Aksel S., Abee C. R. (1983). A pelvimetry method for predicting perinatal mortality in pregnant squirrel monkeys (Saimiri sciuresus). Lab. Anim. Sci. 33, 165–167. - PubMed
-
- Aldunate M., Srbinovski D., Hearps A. C., Latham C. F., Ramsland P. A., Gugasyan R., et al. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6:164. 10.3389/fphys.2015.00164 - DOI - PMC - PubMed
-
- Altizer S. M., Nunn C. L., Thrall P. H., Gittleman J. L., Antonovics J., Cunningham A. A., et al. (2003). Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 34, 517–547. 10.1146/annurev.ecolsys.34.030102.151725 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

