Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results
- PMID: 28008555
- PMCID: PMC5241330
- DOI: 10.1007/s10549-016-4085-4
Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results
Abstract
Purpose: Genetic predisposition to male breast cancer (MBC) is not well understood. The aim of this study was to better define the predisposition genes contributing to MBC and the utility of germline multi-gene panel testing (MGPT) for explaining the etiology of MBCs.
Methods: Clinical histories and molecular results were retrospectively reviewed for 715 MBC patients who underwent MGPT from March 2012 to June 2016.
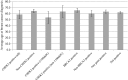
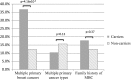
Results: The detection rate of MGPT was 18.1% for patients tested for variants in 16 breast cancer susceptibility genes and with no prior BRCA1/2 testing. BRCA2 and CHEK2 were the most frequently mutated genes (11.0 and 4.1% of patients with no prior BRCA1/2 testing, respectively). Pathogenic variants in BRCA2 [odds ratio (OR) = 13.9; p = 1.92 × 10-16], CHEK2 (OR = 3.7; p = 6.24 × 10-24), and PALB2 (OR = 6.6, p = 0.01) were associated with significantly increased risks of MBC. The average age at diagnosis of MBC was similar for patients with (64 years) and without (62 years) pathogenic variants. CHEK2 1100delC carriers had a significantly lower average age of diagnosis (n = 7; 54 years) than all others with pathogenic variants (p = 0.03). No significant differences were observed between history of additional primary cancers (non-breast) and family history of male breast cancer for patients with and without pathogenic variants. However, patients with pathogenic variants in BRCA2 were more likely to have a history of multiple primary breast cancers.
Conclusion: These data suggest that all MBC patients regardless of age of diagnosis, history of multiple primary cancers, or family history of MBC should be offered MGPT.
Keywords: BRCA2; CHEK2; Male breast cancer; Multi-gene panel testing; PALB2.
Conflict of interest statement
Mary Pritzlaff, Pia Summerour, Shuwei Li, Patrick Reineke, Jill S. Dolinsky, Rachel McFarland, and Holly LaDuca are employees of Ambry Genetics. Jill S. Dolinsky and Elizabeth Chao are stock holders of Ambry Genetics. The other authors have nothing to disclose. Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The research presented here complies with the current laws of the United States of America. Research involving human and animal rights This article does not contain any studies with animals performed by any of the authors.
Figures
References
-
- The NCCN Clinical Practice Guidelines in Oncology™ Genetic/Familial High-Risk Assessment: Breast and Ovarian V2.2016. National Comprehensive Cancer Network, Inc. 2016. http://www.nccn.org/. Accessed 22 Sept 2016
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous