Use of Tumor Markers in Gastrointestinal Cancers: Surgeon Perceptions and Cost-Benefit Trade-Off Analysis
- PMID: 28008574
- PMCID: PMC5374165
- DOI: 10.1245/s10434-016-5717-y
Use of Tumor Markers in Gastrointestinal Cancers: Surgeon Perceptions and Cost-Benefit Trade-Off Analysis
Abstract
Background: Gastrointestinal cancers constitute the third most common cancers worldwide. Tumor markers have long since been used in the postoperative surveillance of these malignancies; however, the true value in clinical practice remains undetermined.
Objective: This study aimed to evaluate the clinical utility of three tumor markers in colorectal and esophagogastric cancer.
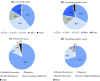
Methods: A systematic review of the literature was undertaken to elicit the sensitivity, specificity, statistical heterogeneity and ability to predict recurrence and metastases for carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9 and CA125. European surgeons were surveyed to assess their current practice and the characteristics of tumor markers they most valued. Data from the included studies and survey were combined in a cost-benefit trade-off analysis to assess which tumor markers are of most use in clinical practice.
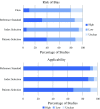
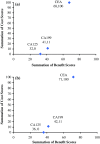
Results: Diagnostic sensitivity and specificity were ranked the most desirable characteristics of a tumor marker by those surveyed. Overall, 156 studies were included to inform the cost-benefit trade-off. The cost-benefit trade-off showed that CEA outperformed both CA19-9 and CA125, with lower financial cost and a higher sensitivity, and diagnostic accuracy for metastases at presentation (area under the curve [AUC] 0.70 vs. 0.61 vs. 0.46), as well as similar diagnostic accuracy for recurrence (AUC 0.46 vs. 0.48).
Conclusions: Cost-benefit trade-off analysis identified CEA to be the best performing tumor marker. Further studies should seek to evaluate new tumor markers, with investigation tailored to factors that meet the requirements of practicing clinicians.
Figures
References
-
- National Cancer Institute. SEER stat fact sheets. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed 1 Nov 2015.
-
- Carpelan-Holmstrom M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22(4):2311–2316. - PubMed
-
- Yang XQ, Chen C, Wang FB, Peng CW, Li Y. Preoperative serum carcinoembryonic antigen, carbohydrate antigen19-9 and carbohydrate antigen 125 as prognostic factors for recurrence-free survival in colorectal cancer. Asian Pac J Cancer Prev. 2011;12(5):1251–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

