Defective expression of apoptosis-related molecules in multiple sclerosis patients is normalized early after autologous haematopoietic stem cell transplantation
- PMID: 28008595
- PMCID: PMC5290242
- DOI: 10.1111/cei.12895
Defective expression of apoptosis-related molecules in multiple sclerosis patients is normalized early after autologous haematopoietic stem cell transplantation
Abstract
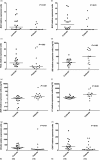
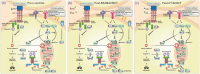
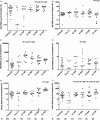
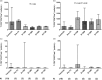
Defective apoptosis might be involved in the pathogenesis of multiple sclerosis (MS). We evaluated apoptosis-related molecules in MS patients before and after autologous haematopoietic stem cell transplantation (AHSCT) using BCNU, Etoposide, AraC and Melphalan (BEAM) or cyclophosphamide (CY)-based conditioning regimens. Patients were followed for clinical and immunological parameters for 2 years after AHSCT. At baseline, MS patients had decreased proapoptotic BAD, BAX and FASL and increased A1 gene expression when compared with healthy counterparts. In the BEAM group, BAK, BIK, BIMEL , FAS, FASL, A1, BCL2, BCLXL , CFLIPL and CIAP2 genes were up-regulated after AHSCT. With the exception of BIK, BIMEL and A1, all genes reached levels similar to controls at day + 720 post-transplantation. Furthermore, in these patients, we observed increased CD8+ Fas+ T cell frequencies after AHSCT when compared to baseline. In the CY group, we observed increased BAX, BCLW, CFLIPL and CIAP1 and decreased BIK and BID gene expressions after transplantation. At day + 720 post-AHSCT, the expression of BAX, FAS, FASL, BCL2, BCLXL and CIAP1 was similar to that of controls. Protein analyses showed increased Bcl-2 expression before transplantation. At 1 year post-AHSCT, expression of Bak, Bim, Bcl-2, Bcl-xL and cFlip-L was decreased when compared to baseline values. In summary, our findings suggest that normalization of apoptosis-related molecules is associated with the early therapeutic effects of AHSCT in MS patients. These mechanisms may be involved in the re-establishment of immune tolerance during the first 2 years post-transplantation.
Keywords: apoptosis-related molecules; autologous haematopoietic stem cell transplantation; autoreactive cells; immune tolerance; multiple sclerosis.
© 2016 British Society for Immunology.
Figures

References
-
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol 2015; 15:545–58. - PubMed
-
- Stys PK, Zamponi GW, Van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012; 13:507–14. - PubMed
-
- Hafler DA, Compston A, Sawcer S et al Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007; 357:851–62. - PubMed
-
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: The role of infection. Ann Neurol 2007; 61:288–99. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

