High levels of iron supplementation prevents neural tube defects in the Fpn1ffe mouse model
- PMID: 28008752
- PMCID: PMC5388561
- DOI: 10.1002/bdra.23542
High levels of iron supplementation prevents neural tube defects in the Fpn1ffe mouse model
Abstract
Background: Periconception maternal nutrition and folate in particular are important factors influencing the incidence of neural tube defects (NTDs). Many but not all NTDs are prevented by folic acid supplementation and there is a pressing need for additional strategies to prevent these birth defects. Other micronutrients such as iron are potential candidates, yet a clear role for iron deficiency in contributing to NTDs is lacking. Our previous studies with the flatiron (ffe) mouse model of Ferroportin1 (Fpn1) deficiency suggest that iron is required for neural tube closure and forebrain development raising the possibility that iron supplementation could prevent NTDs.
Methods: We determined the effect of periconception iron and/or folic acid supplementation on the penetrance of NTDs in the Fpn1ffe mouse model. Concurrently, measurements of folate and iron were made to ensure supplementation had the intended effects.
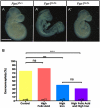
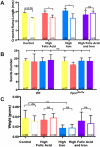
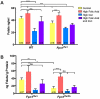
Results: High levels of iron supplementation significantly reduced the incidence of NTDs in Fpn1ffe mutants. Fpn1 deficiency resulted in reduced folate levels in both pregnant dams and embryos. Yet folic acid supplementation did not prevent NTDs in the Fpn1ffe model. Similarly, forebrain truncations were rescued with iron. Surprisingly, the high levels of iron supplementation used in this study caused folate deficiency in wild-type dams and embryos.
Conclusion: Our results demonstrate that iron supplementation can prevent NTDs and forebrain truncations in the Fpn1ffe model. Surprisingly, high levels of iron supplementation and iron overload can cause folate deficiency. If iron is essential for neural tube closure, it is possible that iron deficiency might contribute to NTDs. Birth Defects Research 109:81-91, 2017. © 2016 The Authors Birth Defects Research Published by Wiley Periodicals, Inc.
Keywords: exencephaly; folic acid supplementation; iron deficiency; neural tube defects; spina bifida.
© 2016 The Authors Birth Defects Research Published by Wiley Periodicals, Inc.
Figures
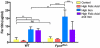

References
-
- Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, Simeone A. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125:5091–5104. - PubMed
-
- Arakawa T, Ohara K, Kakizaki R, Takahashi Y, Hirata K, Fujii M, Konno T, Morikawa T, Chiba F, Chiba R. Folic acid deficiency in hemochromatosis: probably due to a defective storage of folic acid in the liver. The Tohoku journal of experimental medicine. 1965;86:301–306. - PubMed
-
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. The American journal of clinical nutrition. 2000;72:257S–264S. - PubMed
-
- Buamah PK, Russell M, Bates G, Ward AM, Skillen AW. Maternal zinc status: a determination of central nervous system malformation. British journal of obstetrics and gynaecology. 1984;91:788–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

