Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells
- PMID: 28008958
- PMCID: PMC5180086
- DOI: 10.1038/srep39775
Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells
Abstract
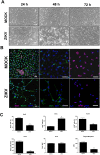
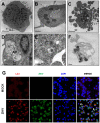
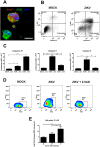
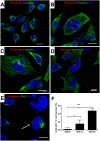
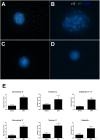
Zika virus (ZIKV) infection has been associated with severe complications both in the developing and adult nervous system. To investigate the deleterious effects of ZIKV infection, we used human neural progenitor cells (NPC), derived from induced pluripotent stem cells (iPSC). We found that NPC are highly susceptible to ZIKV and the infection results in cell death. ZIKV infection led to a marked reduction in cell proliferation, ultrastructural alterations and induction of autophagy. Induction of apoptosis of Sox2+ cells was demonstrated by activation of caspases 3/7, 8 and 9, and by ultrastructural and flow cytometry analyses. ZIKV-induced death of Sox2+ cells was prevented by incubation with the pan-caspase inhibitor, Z-VAD-FMK. By confocal microscopy analysis we found an increased number of cells with supernumerary centrosomes. Live imaging showed a significant increase in mitosis abnormalities, including multipolar spindle, chromosome laggards, micronuclei and death of progeny after cell division. FISH analysis for chromosomes 12 and 17 showed increased frequency of aneuploidy, such as monosomy, trisomy and polyploidy. Our study reinforces the link between ZIKV and abnormalities in the developing human brain, including microcephaly.
Figures

References
-
- Dick G. W., Kitchen S. F. & Haddow A. J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg 46, 509–520 (1952). - PubMed
-
- Duffy M. R. et al.. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med 360, 2536–2543 (2009). - PubMed
-
- Hennessey M., Fischer M. & Staples J. E. Zika Virus Spreads to New Areas - Region of the Americas, May 2015-January 2016. MMWR Morb. Mortal. Wkly. Rep. 65, 55–58 (2016). - PubMed
-
- Kleber de Oliveira W. et al.. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 65, 242–247 (2016). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

