Non-invasive imaging using reporter genes altering cellular water permeability
- PMID: 28008959
- PMCID: PMC5196229
- DOI: 10.1038/ncomms13891
Non-invasive imaging using reporter genes altering cellular water permeability
Abstract
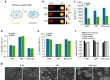
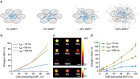
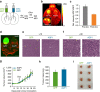
Non-invasive imaging of gene expression in live, optically opaque animals is important for multiple applications, including monitoring of genetic circuits and tracking of cell-based therapeutics. Magnetic resonance imaging (MRI) could enable such monitoring with high spatiotemporal resolution. However, existing MRI reporter genes based on metalloproteins or chemical exchange probes are limited by their reliance on metals or relatively low sensitivity. Here we introduce a new class of MRI reporters based on the human water channel aquaporin 1. We show that aquaporin overexpression produces contrast in diffusion-weighted MRI by increasing tissue water diffusivity without affecting viability. Low aquaporin levels or mixed populations comprising as few as 10% aquaporin-expressing cells are sufficient to produce MRI contrast. We characterize this new contrast mechanism through experiments and simulations, and demonstrate its utility in vivo by imaging gene expression in tumours. Our results establish an alternative class of sensitive, metal-free reporter genes for non-invasive imaging.
Figures

References
-
- Rao J., Dragulescu-Andrasi A. & Yao H. Fluorescence imaging in vivo: recent advances. Curr. Opin. Biotechnol. 18, 17–25 (2007). - PubMed
-
- Contag C. H. & Bachmann M. H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4, 235–260 (2002). - PubMed
-
- Chudakov D. M., Matz M. V., Lukyanov S. & Lukyanov K. A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 90, 1103–1163 (2010). - PubMed
-
- Ntziachristos V., Ripoll J., Wang L. V. & Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat. Biotechnol. 23, 313–320 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

