Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity
- PMID: 28008980
- PMCID: PMC5180178
- DOI: 10.1038/srep39738
Antiretroviral Treatment with Efavirenz Disrupts the Blood-Brain Barrier Integrity and Increases Stroke Severity
Abstract
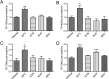
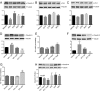
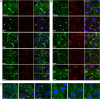
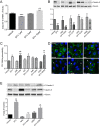
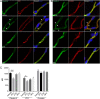
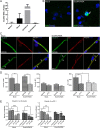
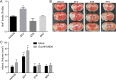
The introduction of antiretroviral drugs (ARVd) changed the prognosis of HIV infection from a deadly disease to a chronic disease. However, even with undetectable viral loads, patients still develop a wide range of pathologies, including cerebrovascular complications and stroke. It is hypothesized that toxic side effects of ARVd may contribute to these effects. To address this notion, we evaluated the impact of several non-nucleoside reverse transcriptase inhibitors (NNRTI; Efavirenz, Etravirine, Rilpivirine and Nevirapine) on the integrity of the blood-brain barrier, and their impact on severity of stroke. Among studied drugs, Efavirenz, but not other NNRTIs, altered claudin-5 expression, increased endothelial permeability, and disrupted the blood-brain barrier integrity. Importantly, Efavirenz exposure increased the severity of stroke in a model of middle cerebral artery occlusion in mice. Taken together, these results indicate that selected ARVd can exacerbate HIV-associated cerebrovascular pathology. Therefore, careful consideration should be taken when choosing an anti-retroviral therapy regimen.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

