Translational control of auditory imprinting and structural plasticity by eIF2α
- PMID: 28009255
- PMCID: PMC5245967
- DOI: 10.7554/eLife.17197
Translational control of auditory imprinting and structural plasticity by eIF2α
Abstract
The formation of imprinted memories during a critical period is crucial for vital behaviors, including filial attachment. Yet, little is known about the underlying molecular mechanisms. Using a combination of behavior, pharmacology, in vivo surface sensing of translation (SUnSET) and DiOlistic labeling we found that, translational control by the eukaryotic translation initiation factor 2 alpha (eIF2α) bidirectionally regulates auditory but not visual imprinting and related changes in structural plasticity in chickens. Increasing phosphorylation of eIF2α (p-eIF2α) reduces translation rates and spine plasticity, and selectively impairs auditory imprinting. By contrast, inhibition of an eIF2α kinase or blocking the translational program controlled by p-eIF2α enhances auditory imprinting. Importantly, these manipulations are able to reopen the critical period. Thus, we have identified a translational control mechanism that selectively underlies auditory imprinting. Restoring translational control of eIF2α holds the promise to rejuvenate adult brain plasticity and restore learning and memory in a variety of cognitive disorders.
Keywords: behavior; chicken; critical period; dendritic spines; early learning; imprinting; memory formation; neuroethology; neuroscience; structural plasticity; translational control.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






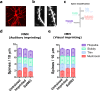
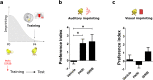

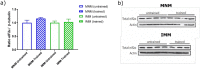
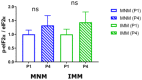
References
-
- Bock J, Schnabel R, Braun K. Role of the dorso-caudal neostriatum in filial imprinting of the domestic chick: a pharmacological and autoradiographical approach focused on the involvement of NMDA-receptors. European Journal of Neuroscience. 1997;9:1262–1272. doi: 10.1111/j.1460-9568.1997.tb01481.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

