Interneuronal mechanisms of hippocampal theta oscillations in a full-scale model of the rodent CA1 circuit
- PMID: 28009257
- PMCID: PMC5313080
- DOI: 10.7554/eLife.18566
Interneuronal mechanisms of hippocampal theta oscillations in a full-scale model of the rodent CA1 circuit
Abstract
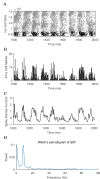
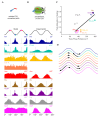
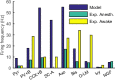
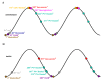
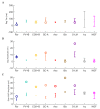
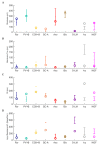
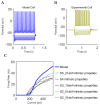
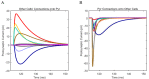
The hippocampal theta rhythm plays important roles in information processing; however, the mechanisms of its generation are not well understood. We developed a data-driven, supercomputer-based, full-scale (1:1) model of the rodent CA1 area and studied its interneurons during theta oscillations. Theta rhythm with phase-locked gamma oscillations and phase-preferential discharges of distinct interneuronal types spontaneously emerged from the isolated CA1 circuit without rhythmic inputs. Perturbation experiments identified parvalbumin-expressing interneurons and neurogliaform cells, as well as interneuronal diversity itself, as important factors in theta generation. These simulations reveal new insights into the spatiotemporal organization of the CA1 circuit during theta oscillations.
Keywords: computational; computational biology; hippocampus; inhibition; model network; mouse; neuroscience; oscillation; rat; systems biology; theta.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

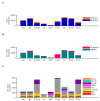
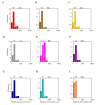

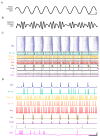
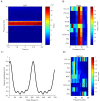
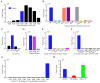

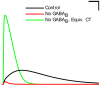
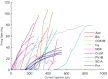
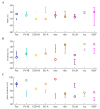
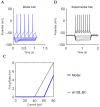
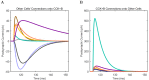
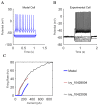
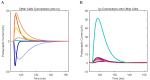

References
-
- Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. USA: Oxford University Press; 2006.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

