Calcium Release from Stores Inhibits GIRK
- PMID: 28009293
- PMCID: PMC5207023
- DOI: 10.1016/j.celrep.2016.11.076
Calcium Release from Stores Inhibits GIRK
Abstract
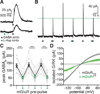
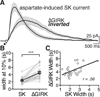
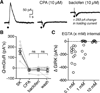
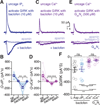
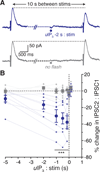
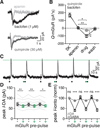
Synaptic transmission is mediated by ionotropic and metabotropic receptors that together regulate the rate and pattern of action potential firing. Metabotropic receptors can activate ion channels and modulate other receptors and channels. The present paper examines the interaction between group 1 mGluR-mediated calcium release from stores and GABAB/D2-mediated GIRK currents in rat dopamine neurons of the Substantia Nigra. Transient activation of mGluRs decreased the GIRK current evoked by GABAB and D2 receptors, although less efficaciously for D2. The mGluR-induced inhibition of GIRK current peaked in 1 s and recovered to baseline after 5 s. The inhibition was dependent on release of calcium from stores, was larger for transient than for tonic currents, and was unaffected by inhibitors of PLC, PKC, PLA2, or calmodulin. This inhibition of GABAB IPSCs through release of calcium from stores is a postsynaptic mechanism that may broadly reduce GIRK-dependent inhibition of many central neurons.
Keywords: D2; GABA(B); GIRK inhibition; IP(3); calcium; dopamine neurons; group I mGluR.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Arora D, Lujan R, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Wickman K. Acute Cocaine Exposure Weakens GABAB Receptor-Dependent G-Protein-Gated Inwardly Rectifying K+ Signaling in Dopamine Neurons of the Ventral Tegmental Area. Journal of Neuroscience. 2011;31:12251–12257. - PMC - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

