Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells
- PMID: 28009296
- PMCID: PMC5217294
- DOI: 10.1016/j.celrep.2016.11.072
Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells
Abstract
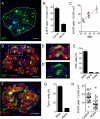
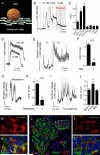
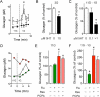
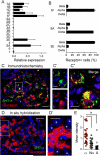
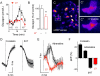
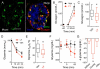
In the pancreatic islet, serotonin is an autocrine signal increasing beta cell mass during metabolic challenges such as those associated with pregnancy or high-fat diet. It is still unclear whether serotonin is relevant for regular islet physiology and hormone secretion. Here, we show that human beta cells produce and secrete serotonin when stimulated with increases in glucose concentration. Serotonin secretion from beta cells decreases cyclic AMP (cAMP) levels in neighboring alpha cells via 5-HT1F receptors and inhibits glucagon secretion. Without serotonergic input, alpha cells lose their ability to regulate glucagon secretion in response to changes in glucose concentration, suggesting that diminished serotonergic control of alpha cells can cause glucose blindness and the uncontrolled glucagon secretion associated with diabetes. Supporting this model, pharmacological activation of 5-HT1F receptors reduces glucagon secretion and has hypoglycemic effects in diabetic mice. Thus, modulation of serotonin signaling in the islet represents a drug intervention opportunity.
Keywords: alpha cell; beta cell; diabetes; glucagon secretion; insulin secretion; pancreatic islet; paracrine signal; serotonin.
Published by Elsevier Inc.
Figures
References
-
- Anlauf M, et al. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:1027–1040. - PubMed
-
- Bennet H, et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides. 2015;71:113–120. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

