Anti-CD22 and anti-CD79b antibody-drug conjugates preferentially target proliferating B cells
- PMID: 28009435
- PMCID: PMC5368047
- DOI: 10.1111/bph.13697
Anti-CD22 and anti-CD79b antibody-drug conjugates preferentially target proliferating B cells
Abstract
Background and purpose: CD22 and CD79b are cell-surface receptors expressed on B-cell-derived malignancies such as non-Hodgkin's lymphoma (NHL). An anti-mitotic agent, monomethyl auristatin E, was conjugated to anti-CD22 and anti-CD79b antibodies to develop target-specific therapies for NHL. The mechanism of action (MOA) and pharmacological and pharmacokinetic (PK) profiles of these antibody-drug conjugates (ADCs) were investigated in cynomolgus monkeys.
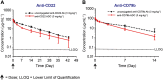
Experimental approach: Animals were administered anti-CD22 or anti-CD79b ADCs, respective unconjugated antibodies or vehicle. Pharmacodynamic effects on total and proliferating B cells and serum PK were then assessed. Antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) of the ADCs were evaluated in vitro.
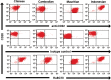
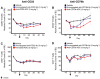
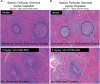
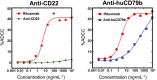
Key results: Depletion of B cells was observed after administration of either ADC or the respective unconjugated antibodies. An extended duration of depletion was observed in animals administered ADCs. Similarly, preferential depletion of proliferating B cells in blood and germinal centre B cells in spleen were only observed in animals administered ADCs. Serum PK profiles of ADCs and respective unconjugated antibodies were comparable. In vitro, anti-human CD22 and anti-human CD79b antibodies showed no or only moderate ADCC activity, respectively; neither antibody had CDC activity.
Conclusions and implications: The findings support the proposed MOA: initial depletion of total B cells by antibody-mediated opsonization, followed by preferential, sustained depletion of proliferating B cells by the auristatin conjugate due to its anti-mitotic action. Delivering potent anti-mitotic agents to B cells via the specificity of monoclonal antibodies provides a means to eliminate pathogenic B cells in NHL with improved risk-benefit profiles over traditional chemotherapeutics.
© 2016 Genentech. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures

Similar articles
-
In vivo effects of targeting CD79b with antibodies and antibody-drug conjugates.Mol Cancer Ther. 2009 Oct;8(10):2937-46. doi: 10.1158/1535-7163.MCT-09-0369. Epub 2009 Oct 6. Mol Cancer Ther. 2009. PMID: 19808977
-
Evaluation and use of an anti-cynomolgus monkey CD79b surrogate antibody-drug conjugate to enable clinical development of polatuzumab vedotin.Br J Pharmacol. 2019 Oct;176(19):3805-3818. doi: 10.1111/bph.14784. Epub 2019 Aug 24. Br J Pharmacol. 2019. PMID: 31270798 Free PMC article.
-
A Novel Anti-CD22 Anthracycline-Based Antibody-Drug Conjugate (ADC) That Overcomes Resistance to Auristatin-Based ADCs.Clin Cancer Res. 2015 Jul 15;21(14):3298-306. doi: 10.1158/1078-0432.CCR-14-2035. Epub 2015 Apr 3. Clin Cancer Res. 2015. PMID: 25840969
-
Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies.Mol Immunol. 2015 Oct;67(2 Pt A):107-16. doi: 10.1016/j.molimm.2014.09.014. Epub 2014 Oct 7. Mol Immunol. 2015. PMID: 25304309 Review.
-
[Immunotherapy of non-Hodgkin's lymphomas (NHL) by anti-CD22 antibody--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Dec;14(6):1258-61. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 17204206 Review. Chinese.
Cited by
-
Let's Talk About BiTEs and Other Drugs in the Real-Life Setting for B-Cell Acute Lymphoblastic Leukemia.Front Immunol. 2019 Dec 20;10:2856. doi: 10.3389/fimmu.2019.02856. eCollection 2019. Front Immunol. 2019. PMID: 31921126 Free PMC article. Review.
-
Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion.Nat Rev Clin Oncol. 2022 May;19(5):342-355. doi: 10.1038/s41571-022-00607-3. Epub 2022 Mar 22. Nat Rev Clin Oncol. 2022. PMID: 35318469 Review.
-
Prediction of Human Pharmacokinetics of Antibody-Drug Conjugates From Nonclinical Data.Clin Transl Sci. 2019 Sep;12(5):534-544. doi: 10.1111/cts.12649. Epub 2019 Jun 11. Clin Transl Sci. 2019. PMID: 31115997 Free PMC article.
-
Pharmacokinetics of protein and peptide conjugates.Drug Metab Pharmacokinet. 2019 Feb;34(1):42-54. doi: 10.1016/j.dmpk.2018.11.001. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30573392 Free PMC article. Review.
-
The Properties of Cysteine-Conjugated Antibody-Drug Conjugates Are Impacted by the IgG Subclass.AAPS J. 2018 Sep 25;20(6):103. doi: 10.1208/s12248-018-0263-0. AAPS J. 2018. PMID: 30255287
References
-
- Advani RH, Lebovic D, Chen A, Brunvand M, Goy A, Chang JE et al. (2016). Phase I study of the anti‐CD22 antibody‐drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B‐cell non‐Hodgkin's lymphoma. Clin Cancer Res. doi:10.1158/1078‐0432.CCR‐16‐0772. - DOI - PMC - PubMed
-
- Arrowsmith J (2011). Trial watch: phase III and submission failures: 2007–2010. Nat Rev Drug Discov 10: 87. - PubMed
-
- Arrowsmith J, Miller P (2013). Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov 12: 569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

