Renal Allograft Survival in Nonhuman Primates Infused With Donor Antigen-Pulsed Autologous Regulatory Dendritic Cells
- PMID: 28009481
- PMCID: PMC5444942
- DOI: 10.1111/ajt.14182
Renal Allograft Survival in Nonhuman Primates Infused With Donor Antigen-Pulsed Autologous Regulatory Dendritic Cells
Abstract
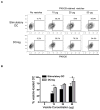
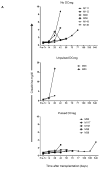
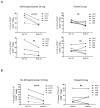
Systemic administration of autologous regulatory dendritic cells (DCreg; unpulsed or pulsed with donor antigen [Ag]), prolongs allograft survival and promotes transplant tolerance in rodents. Here, we demonstrate that nonhuman primate (NHP) monocyte-derived DCreg preloaded with cell membrane vesicles from allogeneic peripheral blood mononuclear cells induce T cell hyporesponsiveness to donor alloantigen (alloAg) in vitro. These donor alloAg-pulsed autologous DCreg (1.4-3.6 × 106 /kg) were administered intravenously, 1 day before MHC-mismatched renal transplantation to rhesus monkeys treated with costimulation blockade (cytotoxic T lymphocyte Ag 4 immunoglobulin [CTLA4] Ig) and tapered rapamycin. Prolongation of graft median survival time from 39.5 days (no DCreg infusion; n = 6 historical controls) and 29 days with control unpulsed DCreg (n = 2), to 56 days with donor Ag-pulsed DCreg (n = 5) was associated with evidence of modulated host CD4+ and CD8+ T cell responses to donor Ag and attenuation of systemic IL-17 production. Circulating anti-donor antibody (Ab) was not detected until CTLA4 Ig withdrawal. One monkey treated with donor Ag-pulsed DCreg rejected its graft in association with progressively elevated anti-donor Ab, 525 days posttransplant (160 days after withdrawal of immunosuppression). These findings indicate a modest but not statistically significant beneficial effect of donor Ag-pulsed autologous DCreg infusion on NHP graft survival when administered with a minimal immunosuppressive drug regimen.
Keywords: T cell biology; animal models: nonhuman primate; basic (laboratory) research/science; dendritic cell; fusion proteins and monoclonal antibodies: belatacept; immunobiology; immunosuppressant; immunosuppression/immune modulation; kidney transplantation/nephrology; mechanistic target of rapamycin (mTOR); translational research/science.
© 2016 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. AWT is co-inventor of a US patent for generation of dendritic cells to promote organ transplant survival. The other authors have no conflicts of interest to disclose.
Figures
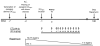







References
-
- Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. - PubMed
-
- Lombardi G, Sagoo P, Scotta C, Fazekasova H, Smyth L, Tsang J, et al. Cell therapy to promote transplantation tolerance: a winning strategy? Immunotherapy. 2011;3(4 Suppl):28–31. - PubMed
-
- Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632–643. - PubMed
-
- Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187(5):2072–2078. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

