Conformational variation of proteins at room temperature is not dominated by radiation damage
- PMID: 28009548
- PMCID: PMC5182021
- DOI: 10.1107/S1600577516017343
Conformational variation of proteins at room temperature is not dominated by radiation damage
Abstract
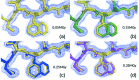
Protein crystallography data collection at synchrotrons is routinely carried out at cryogenic temperatures to mitigate radiation damage. Although damage still takes place at 100 K and below, the immobilization of free radicals increases the lifetime of the crystals by approximately 100-fold. Recent studies have shown that flash-cooling decreases the heterogeneity of the conformational ensemble and can hide important functional mechanisms from observation. These discoveries have motivated increasing numbers of experiments to be carried out at room temperature. However, the trade-offs between increased risk of radiation damage and increased observation of alternative conformations at room temperature relative to cryogenic temperature have not been examined. A considerable amount of effort has previously been spent studying radiation damage at cryo-temperatures, but the relevance of these studies to room temperature diffraction is not well understood. Here, the effects of radiation damage on the conformational landscapes of three different proteins (T. danielli thaumatin, hen egg-white lysozyme and human cyclophilin A) at room (278 K) and cryogenic (100 K) temperatures are investigated. Increasingly damaged datasets were collected at each temperature, up to a maximum dose of the order of 107 Gy at 100 K and 105 Gy at 278 K. Although it was not possible to discern a clear trend between damage and multiple conformations at either temperature, it was observed that disorder, monitored by B-factor-dependent crystallographic order parameters, increased with higher absorbed dose for the three proteins at 100 K. At 278 K, however, the total increase in this disorder was only statistically significant for thaumatin. A correlation between specific radiation damage affecting side chains and the amount of disorder was not observed. This analysis suggests that elevated conformational heterogeneity in crystal structures at room temperature is observed despite radiation damage, and not as a result thereof.
Keywords: conformational dynamics; cryo-temperature; radiation damage; room temperature.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

