Enhanced delivery of IL-1 receptor antagonist to the central nervous system as a novel anti-transferrin receptor-IL-1RA fusion reverses neuropathic mechanical hypersensitivity
- PMID: 28009628
- PMCID: PMC5359788
- DOI: 10.1097/j.pain.0000000000000810
Enhanced delivery of IL-1 receptor antagonist to the central nervous system as a novel anti-transferrin receptor-IL-1RA fusion reverses neuropathic mechanical hypersensitivity
Abstract
Neuropathic pain is a major unmet medical need, with only 30% to 35% of patients responding to the current standard of care. The discovery and development of novel therapeutics to address this unmet need have been hampered by poor target engagement, the selectivity of novel molecules, and limited access to the relevant compartments. Biological therapeutics, either monoclonal antibodies (mAbs) or peptides, offer a solution to the challenge of specificity as the intrinsic selectivity of these kinds of molecules is significantly higher than traditional medicinal chemistry-derived approaches. The interleukin-1 receptor system within the spinal cord has been implicated in the amplification of pain signals, and its central antagonism provides relief of neuropathic pain. Targeting the IL-1 system in the spinal cord with biological drugs, however, raises the even greater challenge of delivery to the central compartment. Targeting the transferrin receptor with monoclonal antibodies has proved successful in traversing the endothelial cell-derived blood-brain barrier and delivering proteins to the central nervous system. In this study, we describe a novel construct exemplifying an engineered solution to overcome these challenges. We have generated a novel anti-transferrin receptor-interleukin-1 receptor antagonist fusion that transports to the central nervous system and delivers efficacy in a model of nerve ligation-induced hypersensitivity. Approaches such as these provide promise for novel and selective analgesics that target the central compartment.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
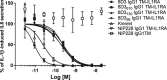
Figures





References
-
- Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. PAIN 2005;116:213–19. - PubMed
-
- Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010;10:345–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

