Multicenter, Prospective, Longitudinal Study of the Recurrence, Surgical Site Infection, and Quality of Life After Contaminated Ventral Hernia Repair Using Biosynthetic Absorbable Mesh: The COBRA Study
- PMID: 28009747
- PMCID: PMC5181129
- DOI: 10.1097/SLA.0000000000001601
Multicenter, Prospective, Longitudinal Study of the Recurrence, Surgical Site Infection, and Quality of Life After Contaminated Ventral Hernia Repair Using Biosynthetic Absorbable Mesh: The COBRA Study
Abstract
Objective: The aim of the study was to evaluate biosynthetic absorbable mesh in single-staged contaminated (Centers for Disease Control class II and III) ventral hernia (CVH) repair over 24 months.
Background: CVH has an increased risk of postoperative infection. CVH repair with synthetic or biologic meshes has reported chronic biomaterial infections and high hernia recurrence rates.
Methods: Patients with a contaminated or clean-contaminated operative field and a hernia defect at least 9 cm had a biosynthetic mesh (open, sublay, retrorectus, or intraperitoneal) repair with fascial closure (n = 104). Endpoints included overall Kaplan-Meier estimates for hernia recurrence and postoperative wound infection rates at 24 months, and the EQ-5D and Short Form 12 Health Survey (SF-12). Analyses were conducted on the intent-to-treat population, and health outcome measures evaluated using paired t tests.
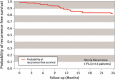
Results: Patients had a mean age of 58 years, body mass index of 28 kg/m, 77% had contaminated wounds, and 84% completed 24-months follow-up. Concomitant procedures included fistula takedown (n = 24) or removal of infected previously placed mesh (n = 29). Hernia recurrence rate was 17% (n = 16). At the time of CVH repair, intraperitoneal placement of the biosynthetic mesh significantly increased the risk of recurrences (P ≤ 0.04). Surgical site infections (19/104) led to higher risk of recurrence (P < 0.01). Mean 24-month EQ-5D (index and visual analogue) and SF-12 physical component and mental scores improved from baseline (P < 0.05).
Conclusions: In this prospective longitudinal study, biosynthetic absorbable mesh showed efficacy in terms of long-term recurrence and quality of life for CVH repair patients and offers an alternative to biologic and permanent synthetic meshes in these complex situations.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

Comment in
-
Questions Regarding Statistical Inferences, Quality of Life, and Conclusions From the COBRA Study.Ann Surg. 2018 Mar;267(3):e58-e59. doi: 10.1097/SLA.0000000000002107. Ann Surg. 2018. PMID: 27930385 Free PMC article. No abstract available.
-
Reply to Letter to The Editor Regarding the COBRA Trial: What Will it Take to Perform High-quality Hernia Research?Ann Surg. 2018 Mar;267(3):e59-e60. doi: 10.1097/SLA.0000000000002108. Ann Surg. 2018. PMID: 28002061 No abstract available.
References
-
- Cobb WS, Carbonell AM, Kalbaugh CL, et al. Infection risk of open placement of intraperitoneal composite mesh. Am Surg 2009; 75:762–767. - PubMed
-
- Hawn MT, Snyder CW, Graham LA, et al. Long-term follow-up of technical outcomes for incisional hernia repair. J Am Coll Surg 2010; 210:648–655. - PubMed
-
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Eng J Med 2000; 343:392–398. - PubMed
-
- Park AE, Roth JS, Kavic SM. Abdominal wall hernia. Curr Probl Surg 2006; 43:326–375. - PubMed
-
- Baillie DR, Stawicki SP, Eustance N, et al. Use of human and porcine dermal-derived bioprostheses in complex abdominal wall reconstructions: a literature review and case report. Ostomy Wound Manage 2007; 53:30–37. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

