Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of p38MAPK
- PMID: 28010122
- PMCID: PMC5563924
- DOI: 10.1089/ars.2016.6642
Lysyl Oxidase Induces Vascular Oxidative Stress and Contributes to Arterial Stiffness and Abnormal Elastin Structure in Hypertension: Role of p38MAPK
Erratum in
-
Correction to: Antioxid Redox Signal 2017; 27(7): 379-397. DOI: 10.1089/ars.2016.6642.Antioxid Redox Signal. 2017 Dec 20;27(18):1520. doi: 10.1089/ars.2016.6642.correx. Epub 2017 Oct 26. Antioxid Redox Signal. 2017. PMID: 29072930 Free PMC article. No abstract available.
Abstract
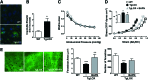
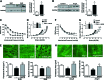
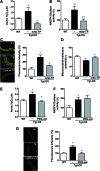
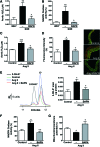
Aims: Vascular stiffness, structural elastin abnormalities, and increased oxidative stress are hallmarks of hypertension. Lysyl oxidase (LOX) is an elastin crosslinking enzyme that produces H2O2 as a by-product. We addressed the interplay between LOX, oxidative stress, vessel stiffness, and elastin.
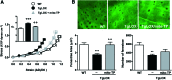
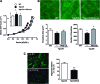
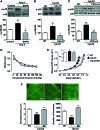
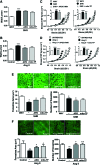
Results: Angiotensin II (Ang II)-infused hypertensive mice and spontaneously hypertensive rats (SHR) showed increased vascular LOX expression and stiffness and an abnormal elastin structure. Mice over-expressing LOX in vascular smooth muscle cells (TgLOX) exhibited similar mechanical and elastin alterations to those of hypertensive models. LOX inhibition with β-aminopropionitrile (BAPN) attenuated mechanical and elastin alterations in TgLOX mice, Ang II-infused mice, and SHR. Arteries from TgLOX mice, Ang II-infused mice, and/or SHR exhibited increased vascular H2O2 and O2.- levels, NADPH oxidase activity, and/or mitochondrial dysfunction. BAPN prevented the higher oxidative stress in hypertensive models. Treatment of TgLOX and Ang II-infused mice and SHR with the mitochondrial-targeted superoxide dismutase mimetic mito-TEMPO, the antioxidant apocynin, or the H2O2 scavenger polyethylene glycol-conjugated catalase (PEG-catalase) reduced oxidative stress, vascular stiffness, and elastin alterations. Vascular p38 mitogen-activated protein kinase (p38MAPK) activation was increased in Ang II-infused and TgLOX mice and this effect was prevented by BAPN, mito-TEMPO, or PEG-catalase. SB203580, the p38MAPK inhibitor, normalized vessel stiffness and elastin structure in TgLOX mice.
Innovation: We identify LOX as a novel source of vascular reactive oxygen species and a new pathway involved in vascular stiffness and elastin remodeling in hypertension.
Conclusion: LOX up-regulation is associated with enhanced oxidative stress that promotes p38MAPK activation, elastin structural alterations, and vascular stiffness. This pathway contributes to vascular abnormalities in hypertension. Antioxid. Redox Signal. 27, 379-397.
Keywords: NADPH oxidases; extracellular matrix; free radicals; microvascular; mitochondria; tissue repair and remodeling.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Aguado A, Galán M, Zhenyukh O, Wiggers GA, Roque FR, Redondo S, Peçanha F, Martín A, Fortuño A, Cachofeiro V, Tejerina T, Salaices M, and Briones AM. Mercury induces proliferation and reduces cell size in vascular smooth muscle cells through MAPK, oxidative stress and cyclooxygenase-2 pathways. Toxicol Appl Pharmacol 268: 188–200, 2013 - PubMed
-
- Arribas SM, Hinek A, and González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther 111: 771–791, 2006 - PubMed
-
- Briones AM. and Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12: 135–142, 2010 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

