Dosing guidance for intravenous colistin in critically-ill patients
- PMID: 28011614
- PMCID: PMC5850520
- DOI: 10.1093/cid/ciw839
Dosing guidance for intravenous colistin in critically-ill patients
Abstract
Background: Intravenous colistin is difficult to use because plasma concentrations for antibacterial effect overlap those causing nephrotoxicity, and there is large inter-patient variability in pharmacokinetics. The aim was to develop dosing algorithms for achievement of a clinically desirable average steady-state plasma colistin concentration (Css,avg) of 2mg/L.
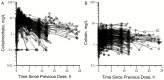
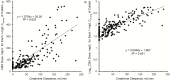
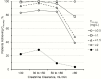
Methods: Plasma concentration-time data from 214 adult critically-ill patients (creatinine clearance 0-236mL/min; 29 receiving renal replacement therapy (RRT)) were subjected to population pharmacokinetic analysis. Development of an algorithm for patients not receiving RRT was based upon the relationship between the dose of colistimethate that would be needed to achieve a desired Css,avg and creatinine clearance. The increase in colistin clearance when patients were on RRT was determined from the population analysis and guided the supplemental dosing needed. To balance potential antibacterial benefit against risk of nephrotoxicity the algorithms were designed to achieve target attainment rates of >80% for Css,avg ≥2 and <30% for Css,avg ≥4mg/L.
Results: When algorithm doses were applied back to individual patients not on RRT (including patients prescribed intermittent dialysis on a non-dialysis day), >80% of patients with creatinine clearance <80mL/min achieved Css,avg ≥2mg/L; but for patients with creatinine clearance ≥80mL/min target attainment was <40%, even with the maximum allowed daily dose of 360mg colistin base activity. For patients receiving RRT, target attainment rates were >80% with the proposed supplemental dosing. In all categories of patients, <30% of patients attained Css,avg ≥4mg/L.
Conclusions: The project has generated clinician-friendly dosing algorithms and pointed to circumstances where intravenous monotherapy may be inadequate.
Keywords: Intravenous colistin; critically-ill patients; dosing guidance; influence of renal impairment and renal replacement modalities; population pharmacokinetics.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Reply to Corona and Cattaneo.Clin Infect Dis. 2017 Sep 1;65(5):870-871. doi: 10.1093/cid/cix390. Clin Infect Dis. 2017. PMID: 29017285 Free PMC article. No abstract available.
-
Dosing Colistin Properly: Let's Save "Our Last Resort Old Drug!".Clin Infect Dis. 2017 Sep 1;65(5):870. doi: 10.1093/cid/cix388. Clin Infect Dis. 2017. PMID: 29017286 No abstract available.
References
-
- Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 2006; 6:589–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

