In Vitro and in Vivo Analyses Reveal Profound Effects of Fibroblast Growth Factor 16 as a Metabolic Regulator
- PMID: 28011645
- PMCID: PMC5290965
- DOI: 10.1074/jbc.M116.751404
In Vitro and in Vivo Analyses Reveal Profound Effects of Fibroblast Growth Factor 16 as a Metabolic Regulator
Abstract
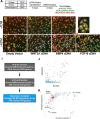
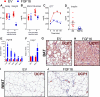
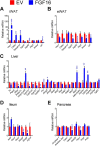
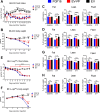
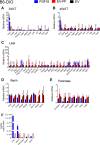
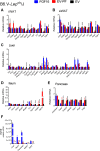
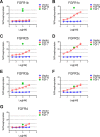
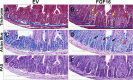
The discovery of brown adipose tissue (BAT) as a key regulator of energy expenditure has sparked interest in identifying novel soluble factors capable of activating inducible BAT (iBAT) to combat obesity. Using a high content cell-based screen, we identified fibroblast growth factor 16 (FGF16) as a potent inducer of several physical and transcriptional characteristics analogous to those of both "classical" BAT and iBAT. Overexpression of Fgf16 in vivo recapitulated several of our in vitro findings, specifically the significant induction of the Ucp1 gene and UCP1 protein expression in inguinal white adipose tissue (iWAT), a common site for emergent active iBAT. Despite significant UCP1 up-regulation in iWAT and dramatic weight loss, the metabolic improvements observed due to Fgf16 overexpression in vivo were not the result of increased energy expenditure, as measured by indirect calorimetric assessment. Instead, a pattern of reduced food and water intake, combined with feces replete with lipid and bile acid, indicated a phenotype more akin to that of starvation and intestinal malabsorption. Gene expression analysis of the liver and ileum indicated alterations in several steps of bile acid metabolism, including hepatic synthesis and reabsorption. Histological analysis of intestinal tissue revealed profound abnormalities in support of this conclusion. The in vivo data, together with FGF receptor binding analysis, indicate that the in vivo outcome observed is the likely result of both direct and indirect mechanisms and probably involves multiple receptors. These results highlight the complexity of FGF signaling in the regulation of various metabolic processes.
Keywords: adipogenesis; bile acid; fibroblast growth factor (FGF); fibroblast growth factor receptor (FGFR); intestinal metabolism; metabolism.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
All authors are or were Amgen employees and thus have an actual or perceived conflict of interest with the contents of this article.
Figures


References
-
- Lowell B. B., and Spiegelman B. M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 - PubMed
-
- Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., and Flier J. S. (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 - PubMed
-
- Feldmann H. M., Golozoubova V., Cannon B., and Nedergaard J. (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

