WIND1 Promotes Shoot Regeneration through Transcriptional Activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis
- PMID: 28011694
- PMCID: PMC5304349
- DOI: 10.1105/tpc.16.00623
WIND1 Promotes Shoot Regeneration through Transcriptional Activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis
Abstract
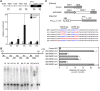
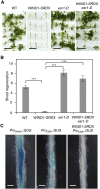
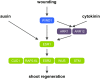
Many plant species display remarkable developmental plasticity and regenerate new organs after injury. Local signals produced by wounding are thought to trigger organ regeneration but molecular mechanisms underlying this control remain largely unknown. We previously identified an AP2/ERF transcription factor WOUND INDUCED DEDIFFERENTIATION1 (WIND1) as a central regulator of wound-induced cellular reprogramming in plants. In this study, we demonstrate that WIND1 promotes callus formation and shoot regeneration by upregulating the expression of the ENHANCER OF SHOOT REGENERATION1 (ESR1) gene, which encodes another AP2/ERF transcription factor in Arabidopsis thaliana The esr1 mutants are defective in callus formation and shoot regeneration; conversely, its overexpression promotes both of these processes, indicating that ESR1 functions as a critical driver of cellular reprogramming. Our data show that WIND1 directly binds the vascular system-specific and wound-responsive cis-element-like motifs within the ESR1 promoter and activates its expression. The expression of ESR1 is strongly reduced in WIND1-SRDX dominant repressors, and ectopic overexpression of ESR1 bypasses defects in callus formation and shoot regeneration in WIND1-SRDX plants, supporting the notion that ESR1 acts downstream of WIND1. Together, our findings uncover a key molecular pathway that links wound signaling to shoot regeneration in plants.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Aida M., Ishida T., Tasaka M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126: 1563–1570. - PubMed
-
- Aida M., Beis D., Heidstra R., Willemsen V., Blilou I., Galinha C., Nussaume L., Noh Y.S., Amasino R., Scheres B. (2004). The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120. - PubMed
-
- Atta R., Laurens L., Boucheron-Dubuisson E., Guivarc’h A., Carnero E., Giraudat-Pautot V., Rech P., Chriqui D. (2009). Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 57: 626–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

