Safetxt: a pilot randomised controlled trial of an intervention delivered by mobile phone to increase safer sex behaviours in young people
- PMID: 28011811
- PMCID: PMC5223743
- DOI: 10.1136/bmjopen-2016-013045
Safetxt: a pilot randomised controlled trial of an intervention delivered by mobile phone to increase safer sex behaviours in young people
Abstract
Objective: To test the procedures proposed for a main trial of a safer sex intervention for young people delivered by mobile phone text message ('safetxt').
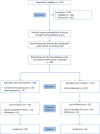
Design and setting: Pilot randomised controlled trial. Participants were recruited through sexual health services in the UK. An independent online randomisation system allocated participants to receive the safetxt intervention or to receive the control text messages (monthly messages about participation in the study). Texting software delivered the messages in accordance with a predetermined schedule.
Participants: Residents of England aged 16-24 who had received either a positive chlamydia test result or reported unsafe sex in the last year (defined as more than 1 partner and at least 1 occasion of sex without a condom).
Intervention: The safetxt intervention is designed to reduce sexually transmitted infection in young people by supporting them in using condoms, telling a partner about an infection and testing before unprotected sex with a new partner. Safetxt was developed drawing on: behavioural science; face-to-face interventions; the factors known to influence safer sex behaviours and the views of young people.
Outcomes: The coprimary outcomes of the pilot trial were the recruitment rate and completeness of follow-up.
Results: We recruited 200 participants within our target of 3 months and we achieved 81% (162/200) follow-up response for the proposed primary outcome of the main trial, cumulative incidence of chlamydia at 12 months.
Conclusions: Recruitment, randomisation, intervention delivery and follow-up were successful and a randomised controlled trial of the safetxt intervention is feasible.
Trial registration number: ISRCTN02304709; Results.
Keywords: INFECTIOUS DISEASES; SEXUAL MEDICINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Conflicts of Interest: None declared.
References
-
- Sonnenberg P, Clifton S, Beddows S et al. . Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382:1795–806. 10.1016/S0140-6736(13)61947-9 - DOI - PMC - PubMed
-
- 2015. The Communications Market Report Ofcom. https://www.ofcom.org.uk/__data/assets/pdf_file/0022/20668/cmr_uk_2015.pdf.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical