Evolution of vocal patterns: tuning hindbrain circuits during species divergence
- PMID: 28011819
- PMCID: PMC6514461
- DOI: 10.1242/jeb.146845
Evolution of vocal patterns: tuning hindbrain circuits during species divergence
Abstract
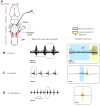
The neural circuits underlying divergent courtship behaviors of closely related species provide a framework for insight into the evolution of motor patterns. In frogs, male advertisement calls serve as unique species identifiers and females prefer conspecific to heterospecific calls. Advertisement calls of three relatively recently (∼8.5 Mya) diverged species - Xenopus laevis, X. petersii and X. victorianus - include rapid trains of sound pulses (fast trills). We show that while fast trills are similar in pulse rate (∼60 pulses s-1) across the three species, they differ in call duration and period (time from the onset of one call to the onset of the following call). Previous studies of call production in X. laevis used an isolated brain preparation in which the laryngeal nerve produces compound action potentials that correspond to the advertisement call pattern (fictive calling). Here, we show that serotonin evokes fictive calling in X. petersii and X. victorianus as it does in X. laevis As in X. laevis, fictive fast trill in X. petersii and X. victorianus is accompanied by an N-methyl-d-aspartate receptor-dependent local field potential wave in a rostral hindbrain nucleus, DTAM. Across the three species, wave duration and period are strongly correlated with species-specific fast trill duration and period, respectively. When DTAM is isolated from the more rostral forebrain and midbrain and/or more caudal laryngeal motor nucleus, the wave persists at species-typical durations and periods. Thus, intrinsic differences within DTAM could be responsible for the evolutionary divergence of call patterns across these related species.
Keywords: Central pattern generator; Communication; Evolution; Motor; Vocalization; Xenopus.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Albersheim-Carter J., Blubaum A., Ballagh I. H., Missaghi K., Siuda E. R., McMurray G., Bass A. H., Dubuc R., Kelley D. B., Schmidt M. F. et al. (2016). Testing the evolutionary conservation of vocal motoneurons in vertebrates. Respir. Physiol. Neurobiol. 224, 2-10. 10.1016/j.resp.2015.06.010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

