Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia
- PMID: 28011841
- PMCID: PMC5841887
- DOI: 10.1093/europace/euw358
Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia
Abstract
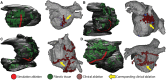
: Atrial arrhythmias involving a fibrotic substrate are an important cause of morbidity and mortality. In many cases, effective treatment of such rhythm disorders is severely hindered by a lack of mechanistic understanding relating features of fibrotic remodelling to dynamics of re-entrant arrhythmia. With the advent of clinical imaging modalities capable of resolving the unique fibrosis spatial pattern present in the atria of each individual patient, a promising new research trajectory has emerged in which personalized computational models are used to analyse mechanistic underpinnings of arrhythmia dynamics based on the distribution of fibrotic tissue. In this review, we first present findings that have yielded a robust and detailed biophysical representation of fibrotic substrate electrophysiological properties. Then, we summarize the results of several recent investigations seeking to use organ-scale models of the fibrotic human atria to derive new insights on mechanisms of arrhythmia perpetuation and to develop novel strategies for model-assisted individualized planning of catheter ablation procedures for atrial arrhythmias.
Keywords: Atrial fibrillation; Atrial flutter; Computational modelling; Fibrotic remodelling.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–68. - PubMed
-
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370–5. - PubMed
-
- Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F. et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. - PubMed
-
- Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A. et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol 2015;26:484–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

