Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection
- PMID: 28011863
- PMCID: PMC5206497
- DOI: 10.1084/jem.20160784
Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection
Abstract
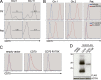
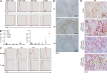
Epstein-Barr virus (EBV) infection in humans is a major trigger of malignant and nonmalignant B cell proliferations. CD27 is a co-stimulatory molecule of T cells, and inherited CD27 deficiency is characterized by high susceptibility to EBV infection, though the underlying pathological mechanisms have not yet been identified. In this study, we report a patient suffering from recurrent EBV-induced B cell proliferations including Hodgkin's lymphoma because of a deficiency in CD70, the ligand of CD27. We show that EBV-specific T lymphocytes did not expand properly when stimulated with CD70-deficient EBV-infected B cells, whereas expression of CD70 in B cells restored expansion, indicating that CD70 on B cells but not on T cells is required for efficient proliferation of T cells. CD70 was found to be up-regulated on B cells when activated and during EBV infection. The proliferation of T cells triggered by CD70-expressing B cells was dependent on CD27 and CD3 on T cells. Importantly, CD27-deficient T cells failed to proliferate when stimulated with CD70-expressing B cells. Thus, the CD70-CD27 pathway appears to be a crucial component of EBV-specific T cell immunity and more generally for the immune surveillance of B cells and may be a target for immunotherapy of B cell malignancies.
© 2017 Izawa et al.
Figures






References
-
- Akiba H., Nakano H., Nishinaka S., Shindo M., Kobata T., Atsuta M., Morimoto C., Ware C.F., Malinin N.L., Wallach D., et al. 1998. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-κB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-κB-inducing kinase. J. Biol. Chem. 273:13353–13358. 10.1074/jbc.273.21.13353 - DOI - PubMed
-
- Alkhairy O.K., Perez-Becker R., Driessen G.J., Abolhassani H., van Montfrans J., Borte S., Choo S., Wang N., Tesselaar K., Fang M., et al. 2015. Novel mutations in TNFRSF7/CD27: Clinical, immunologic, and genetic characterization of human CD27 deficiency. J. Allergy Clin. Immunol. 136:703–712.e10. 10.1016/j.jaci.2015.02.022 - DOI - PubMed
-
- Arens R., Tesselaar K., Baars P.A., van Schijndel G.M., Hendriks J., Pals S.T., Krimpenfort P., Borst J., van Oers M.H., and van Lier R.A.. 2001. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNγ-mediated B cell depletion. Immunity. 15:801–812. 10.1016/S1074-7613(01)00236-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

