Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency
- PMID: 28011864
- PMCID: PMC5206499
- DOI: 10.1084/jem.20160849
Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency
Abstract
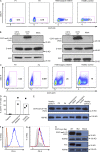
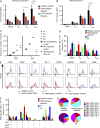
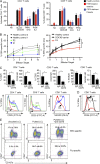
In this study, we describe four patients from two unrelated families of different ethnicities with a primary immunodeficiency, predominantly manifesting as susceptibility to Epstein-Barr virus (EBV)-related diseases. Three patients presented with EBV-associated Hodgkin's lymphoma and hypogammaglobulinemia; one also had severe varicella infection. The fourth had viral encephalitis during infancy. Homozygous frameshift or in-frame deletions in CD70 in these patients abolished either CD70 surface expression or binding to its cognate receptor CD27. Blood lymphocyte numbers were normal, but the proportions of memory B cells and EBV-specific effector memory CD8+ T cells were reduced. Furthermore, although T cell proliferation was normal, in vitro-generated EBV-specific cytotoxic T cell activity was reduced because of CD70 deficiency. This reflected impaired activation by, rather than effects during killing of, EBV-transformed B cells. Notably, expression of 2B4 and NKG2D, receptors implicated in controlling EBV infection, on memory CD8+ T cells from CD70-deficient individuals was reduced, consistent with their impaired killing of EBV-infected cells. Thus, autosomal recessive CD70 deficiency is a novel cause of combined immunodeficiency and EBV-associated diseases, reminiscent of inherited CD27 deficiency. Overall, human CD70-CD27 interactions therefore play a nonredundant role in T and B cell-mediated immunity, especially for protection against EBV and humoral immunity.
© 2017 Abolhassani et al.
Figures

References
-
- Abolhassani H., Wang N., Aghamohammadi A., Rezaei N., Lee Y.N., Frugoni F., Notarangelo L.D., Pan-Hammarström Q., and Hammarström L.. 2014. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. J. Allergy Clin. Immunol. 134:1375–1380. 10.1016/j.jaci.2014.04.042 - DOI - PMC - PubMed
-
- Agematsu K., Nagumo H., Oguchi Y., Nakazawa T., Fukushima K., Yasui K., Ito S., Kobata T., Morimoto C., and Komiyama A.. 1998. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction. Blood. 91:173–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

