Safety and efficacy of obinutuzumab with CHOP or bendamustine in previously untreated follicular lymphoma
- PMID: 28011903
- PMCID: PMC5395117
- DOI: 10.3324/haematol.2016.152272
Safety and efficacy of obinutuzumab with CHOP or bendamustine in previously untreated follicular lymphoma
Abstract
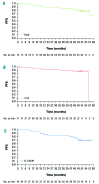
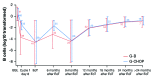
The GAUDI study assessed safety and preliminary efficacy of induction therapy with obinutuzumab plus chemotherapy, followed by maintenance therapy with obinutuzumab alone, in previously untreated patients with follicular lymphoma. Assignment to chemotherapy was decided on a per-center basis before the patients' enrollment. Patients (n=81) received four to six cycles of obinutuzumab plus bendamustine every 4 weeks or six to eight cycles of obinutuzumab plus CHOP every 3 weeks. Patients with an end-of-treatment response were eligible for obinutuzumab maintenance therapy every 3 months for 2 years or until disease progression. Induction treatment was completed by 90% of patients in the obinutuzumab plus bendamustine group and 95% in the obinutuzumab plus CHOP group, while maintenance was completed by 81% and 72% of patients, respectively. All patients experienced at least one adverse event during induction, most commonly infusion-related reactions (58%), the majority of which were grade 1/2. The most common hematologic adverse event was grade 3/4 neutropenia (36% during induction and 7% during maintenance). One treatment-related death occurred during the maintenance phase. At the end of induction, 94% of patients had achieved an overall response, with complete response based on computed tomography in 36%. The progression-free survival rate at 36 months was 90% in the obinutuzumab plus bendamustine group and 84% in the obinutuzumab plus CHOP group. These results demonstrate that induction therapy with obinutuzumab plus bendamustine or obinutuzumab plus CHOP, followed by obinutuzumab maintenance, is associated with tolerable safety and promising efficacy. This study is registered at ClinicalTrials.gov as NCT00825149.
Copyright© Ferrata Storti Foundation.
Figures


References
-
- Dreyling M, Ghielmini M, Marcus R, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii76–iii82. - PubMed
-
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. - PubMed
-
- Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70(11):1445–1476. - PubMed
-
- Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–3837. - PubMed
-
- Heinrich DA, Weinkauf M, Hutter G, et al. Differential regulation patterns of the anti-CD20 antibodies obinutuzumab and rituximab in mantle cell lymphoma. Br J Haematol. 2015;168(4):606–610. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

