The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action
- PMID: 28012107
- PMCID: PMC5313588
- DOI: 10.1007/s00223-016-0220-9
The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action
Abstract
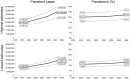
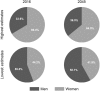
Sarcopenia is a major public health issue. To convince health policy makers of the emergency to invest in the sarcopenia field, it is of critical importance to produce reliable figures of the expected burden of sarcopenia in the coming years. Age- and gender-specific population projections were retrieved until 2045 from the Eurostat online database (28 European countries). Age- and gender-specific prevalences of sarcopenia were interpolated from a study that compared prevalence estimates according to the different diagnostic cutoffs of the EWGSOP proposed definition. The reported prevalence estimates were interpolated between 65 and 100 years. Interpolated age- and gender-specific estimates of sarcopenia prevalence were then applied to population projections until 2045. Using the definition providing the lowest prevalence estimates, the number of individuals with sarcopenia would rise in Europe from 10,869,527 in 2016 to 18,735,173 in 2045 (a 72.4% increase). This corresponds to an overall prevalence of sarcopenia in the elderly rising from 11.1% in 2016 to 12.9% in 2045. With the definition providing the highest prevalence estimates, the number of individuals with sarcopenia would rise from 19,740,527 in 2016 to 32,338,990 in 2045 (a 63.8% increase), corresponding to overall prevalence rates in the elderly of 20.2% and 22.3% for 2016 and 2045, respectively. We showed that the number of sarcopenic patients will dramatically increase in the next 30 years, making consequences of muscle wasting a major public health issue.
Keywords: Burden of disease; Prevalence; Public health; Sarcopenia.
Conflict of interest statement
Conflict of interest
O. Ethgen, C. Beaudart, F. Buckinx, O. Bruyère and J.Y. Reginster declare that they have no conflicts of interest pertaining this particular manuscript.
Human and Animal Rights and Informed Consent
For this type of study formal consent is not required.
Figures


References
-
- Reginster JY, Cooper C, Rizzoli R, Kanis JA, Appelboom G, Bautmans I, Bischoff-Ferrari HA, Boers M, Brandi ML, Bruyere O, Cherubini A, Flamion B, Fielding RA, Gasparik AI, Van Loon L, McCloskey E, Mitlak BH, Pilotto A, Reiter-Niesert S, Rolland Y, Tsouderos Y, Visser M, Cruz-Jentoft AJ. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res. 2016;28:47–58. doi: 10.1007/s40520-015-0517-y. - DOI - PMC - PubMed
-
- Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, Sieber CC, Kaufman JM, Abellan van Kan G, Boonen S, Adachi J, Mitlak B, Tsouderos Y, Rolland Y, Reginster JY. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–1848. doi: 10.1007/s00198-012-1913-1. - DOI - PubMed
-
- Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB, Health ABCSI. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

