Eukaryotic protein glycosylation: a primer for histochemists and cell biologists
- PMID: 28012131
- PMCID: PMC5306191
- DOI: 10.1007/s00418-016-1526-4
Eukaryotic protein glycosylation: a primer for histochemists and cell biologists
Abstract
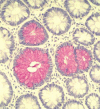
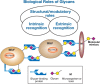
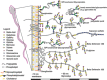
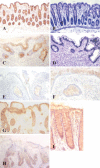
Proteins undergo co- and posttranslational modifications, and their glycosylation is the most frequent and structurally variegated type. Histochemically, the detection of glycan presence has first been performed by stains. The availability of carbohydrate-specific tools (lectins, monoclonal antibodies) has revolutionized glycophenotyping, allowing monitoring of distinct structures. The different types of protein glycosylation in Eukaryotes are described. Following this educational survey, examples where known biological function is related to the glycan structures carried by proteins are given. In particular, mucins and their glycosylation patterns are considered as instructive proof-of-principle case. The tissue and cellular location of glycoprotein biosynthesis and metabolism is reviewed, with attention to new findings in goblet cells. Finally, protein glycosylation in disease is documented, with selected examples, where aberrant glycan expression impacts on normal function to let disease pathology become manifest. The histological applications adopted in these studies are emphasized throughout the text.
Keywords: Eukaryocyte; Glycans; Glycoprotein; Glycosylation; Histochemistry; Mucin.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

