Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy
- PMID: 28012259
- PMCID: PMC5333888
- DOI: 10.1002/hep.28923
Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy
Abstract
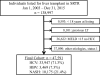
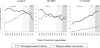
Direct-acting antiviral (DAA) therapy, recently approved for patients with decompensated cirrhosis (DC) secondary to hepatitis C virus (HCV), is associated with improved hepatic function. We analyzed trends in liver transplant (LT) wait-listing (WL) to explore potential impact of effective medical therapy on WL registration. This is a cohort study using the Scientific Registry of Transplant Recipients database from 2003 to 2015. A total of 47,591 adults wait-listed for LT from HCV, hepatitis B virus (HBV), and nonalcoholic steatohepatitis (NASH) were identified. LT indication was defined as DC if the Model for End-Stage Liver Disease (MELD) at WL was ≥15 or hepatocellular carcinoma (HCC). Era of listing was divided into interferon (IFN; 2003-2010), protease inhibitor (PI; 2011-2013), and direct-acting antiviral (DAA; 2014-2015). Annual standardized incidence rates of WL were analyzed using Poisson regression. Adjusted incidences of LT WL for DC in HCV patients decreased by 5% in the PI era (P = 0.004) and 32% in the DAA era (P < 0.001) compared to the IFN era. Listing for DC in HBV also decreased in the PI (-17%; P = 0.002) and DAA eras (-24%; P < 0.001). Conversely, WL for DC in NASH increased by 41% in the PI era (P < 0.001) and 81% in the DAA era (P < 0.001). WL for HCC in both the HCV and NASH populations increased in both the PI and DAA eras (P < 0.001 for all) whereas HCC WL in HBV remained stable (P > 0.05 for all).
Conclusion: The rate of LT WL for HCV complicated by DC has decreased by over 30% in the era of DAA therapy. Further reductions in WL are anticipated with increased testing, linkage to care, and access to DAA therapy. (Hepatology 2017;65:804-812).
© 2016 by the American Association for the Study of Liver Diseases.
Figures

References
-
- Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014;14(Suppl 1):69–96. - PubMed
-
- Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–21. 21, e1–6. - PubMed
-
- van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. - PubMed
-
- Wu CY, Lin JT, Ho HJ, et al. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology. 2014;147(1):143–51. e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

