B cell and antibody responses in mice induced by a putative cell surface peptidase of Pneumocystis murina protect against experimental infection
- PMID: 28012778
- PMCID: PMC5241231
- DOI: 10.1016/j.vaccine.2016.11.073
B cell and antibody responses in mice induced by a putative cell surface peptidase of Pneumocystis murina protect against experimental infection
Abstract
Rationale: Pneumocystis pneumonia is a major cause of morbidity and mortality in HIV-infected subjects, cancer patients undergoing chemotherapy and solid organ transplant recipients. No vaccine is currently available. By chemical labeling coupled with proteomic approach, we have identified a putative surface protein (SPD1, Broad Institute gene accession number PNEG_01848) derived from single suspended P. murina cysts. SPD1 was expressed in an insect cell line and tested for vaccine development.
Methods: Mice were immunized with SPD1 plus adjuvant MF-59 by subcutaneous injection. Three weeks after the last immunization, CD4+ cells were depleted with anti-CD4 antibody GK1.5. The mice were then challenged with 2×105Pneumocystis organisms. Mice were sacrificed at 4 and 6weeks after PC challenge. Spleen/lung cells and serum were harvested. B cells and memory B cells were assessed via flow cytometry. Specific Pneumocystis IgG antibody was measured by ELISA before and after challenge. Infection burden was measured as real-time PCR for P. murina rRNA.
Results: Normal mice infected with Pneumocystis mounted a serum IgG antibody response to SPD1. Serum from rhesus macaques exposed to Pneumocystis showed a similar serum IgG response to purified SPD1. SPD1 immunization increased B cell and memory B cell absolute cell counts in CD4-depleted Balb/c mice post Pneumocystis challenge in spleen and lung. Immunization with SPD1 significantly increased specific Pneumocystis IgG antibody production before and after challenge. Mice immunized with SPD1 showed significantly decreased P. murina copy number compared with mice that did not receive SPD1 at 6weeks after challenge.
Conclusion: Immunization with SPD1 provides protective efficacy against P. murina infection. SPD1 protection against Pneumocystis challenge is associated with enhanced memory B cell production and higher anti-Pneumocystis IgG antibody production. SPD1 is a potential vaccine candidate to prevent or treat pulmonary infection with Pneumocystis.
Keywords: CD4 T-cell deficiency; Pneumocystis pneumonia; Pneumocystis vaccine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
STATEMENT Conflicts of interest: none
Figures





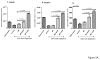
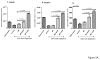

References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, TCW Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. - PubMed
-
- Qian J, Cutler JE, Cole RB, Cai Y. MALDI-TOF mass signatures for differentiation of yeast species, strain grouping and monitoring of morphogenesis markers. Anal Bioanal Chem. 2008;392(3):439–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

