Orbitofrontal participation in sign- and goal-tracking conditioned responses: Effects of nicotine
- PMID: 28012948
- PMCID: PMC5385154
- DOI: 10.1016/j.neuropharm.2016.12.020
Orbitofrontal participation in sign- and goal-tracking conditioned responses: Effects of nicotine
Abstract
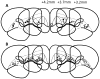
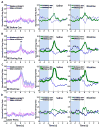
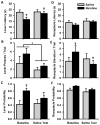
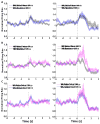
Pavlovian conditioned stimuli can acquire incentive motivational properties, and this phenomenon can be measured in animals using Pavlovian conditioned approach behavior. Drugs of abuse can influence the expression of this behavior, and nicotine in particular exhibits incentive amplifying effects. Both conditioned approach behavior and drug abuse rely on overlapping corticolimbic circuitry. We hypothesize that the orbitofrontal cortex (OFC) regulates conditioned approach, and that one site of nicotine action is in the OFC where it reduces cortical output. To test this, we repeatedly exposed rats to 0.4 mg/kg nicotine (s.c.) during training and then pharmacologically inactivated the lateral OFC or performed in vivo electrophysiological recordings of lateral OFC neurons in the presence or absence of nicotine. In Experiment 1, animals were trained in a Pavlovian conditioning paradigm and behavior was evaluated after inactivation of the OFC by microinfusion of the GABA agonists baclofen and muscimol. In Experiment 2, we monitored phasic firing of OFC neurons during Pavlovian conditioning sessions. Nicotine reliably enhanced conditioned responding to the conditioned cue, and inactivation of the OFC reduced conditioned responding, especially the sign-tracking response. OFC neurons exhibited phasic excitations to cue presentation and during goal tracking, and nicotine acutely blunted this phasic neuronal firing. When nicotine was withheld, both conditioned responding and phasic firing in the OFC returned to the level of controls. These results suggest that the OFC is recruited for the expression of conditioned responses, and that nicotine acutely influences this behavior by reducing phasic firing in the OFC.
Keywords: Electrophysiology; Goal tracking; Inactivation; Nicotine; Orbitofrontal cortex; Rats; Sign tracking.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Notes: The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

