Core-shell microparticles for protein sequestration and controlled release of a protein-laden core
- PMID: 28013102
- PMCID: PMC5478455
- DOI: 10.1016/j.actbio.2016.12.042
Core-shell microparticles for protein sequestration and controlled release of a protein-laden core
Abstract
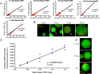
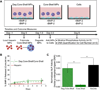
Development of multifunctional biomaterials that sequester, isolate, and redeliver cell-secreted proteins at a specific timepoint may be required to achieve the level of temporal control needed to more fully regulate tissue regeneration and repair. In response, we fabricated core-shell heparin-poly(ethylene-glycol) (PEG) microparticles (MPs) with a degradable PEG-based shell that can temporally control delivery of protein-laden heparin MPs. Core-shell MPs were fabricated via a re-emulsification technique and the number of heparin MPs per PEG-based shell could be tuned by varying the mass of heparin MPs in the precursor PEG phase. When heparin MPs were loaded with bone morphogenetic protein-2 (BMP-2) and then encapsulated into core-shell MPs, degradable core-shell MPs initiated similar C2C12 cell alkaline phosphatase (ALP) activity as the soluble control, while non-degradable core-shell MPs initiated a significantly lower response (85+19% vs. 9.0+4.8% of the soluble control, respectively). Similarly, when degradable core-shell MPs were formed and then loaded with BMP-2, they induced a ∼7-fold higher C2C12 ALP activity than the soluble control. As C2C12 ALP activity was enhanced by BMP-2, these studies indicated that degradable core-shell MPs were able to deliver a bioactive, BMP-2-laden heparin MP core. Overall, these dynamic core-shell MPs have the potential to sequester, isolate, and then redeliver proteins attached to a heparin core to initiate a cell response, which could be of great benefit to tissue regeneration applications requiring tight temporal control over protein presentation.
Statement of significance: Tissue repair requires temporally controlled presentation of potent proteins. Recently, biomaterial-mediated binding (sequestration) of cell-secreted proteins has emerged as a strategy to harness the regenerative potential of naturally produced proteins, but this strategy currently only allows immediate amplification and re-delivery of these signals. The multifunctional, dynamic core-shell heparin-PEG microparticles presented here overcome this limitation by sequestering proteins through a PEG-based shell onto a protein-protective heparin core, temporarily isolating bound proteins from the cellular microenvironment, and re-delivering proteins only after degradation of the PEG-based shell. Thus, these core-shell microparticles have potential to be a novel tool to harness and isolate proteins produced in the cellular environment and then control when proteins are re-introduced for the most effective tissue regeneration and repair.
Keywords: Controlled release; Core-shell microparticles; Heparin; Hydrolytically degradable; Protein delivery.
Copyright © 2016 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures


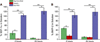

Similar articles
-
Hydrolysis and Sulfation Pattern Effects on Release of Bioactive Bone Morphogenetic Protein-2 from Heparin-Based Microparticles.J Mater Chem B. 2015 Oct 28;3(40):8001-8009. doi: 10.1039/C5TB00933B. Epub 2015 Aug 28. J Mater Chem B. 2015. PMID: 27785363 Free PMC article.
-
Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2.Biomaterials. 2014 Aug;35(25):7228-38. doi: 10.1016/j.biomaterials.2014.05.011. Epub 2014 May 28. Biomaterials. 2014. PMID: 24881028 Free PMC article.
-
Microparticle-mediated sequestration of cell-secreted proteins to modulate chondrocytic differentiation.Acta Biomater. 2018 Mar 1;68:125-136. doi: 10.1016/j.actbio.2017.12.038. Epub 2017 Dec 30. Acta Biomater. 2018. PMID: 29292168 Free PMC article.
-
Design of hydrogels to stabilize and enhance bone morphogenetic protein activity by heparin mimetics.Acta Biomater. 2018 May;72:45-54. doi: 10.1016/j.actbio.2018.03.034. Epub 2018 Mar 26. Acta Biomater. 2018. PMID: 29597024 Free PMC article.
-
The influence of covalently linked and free polyethylene glycol on the structural and release properties of rhBMP-2 loaded microspheres.J Control Release. 2010 Oct 1;147(1):92-100. doi: 10.1016/j.jconrel.2010.06.021. Epub 2010 Jul 29. J Control Release. 2010. PMID: 20603166
Cited by
-
Progress in three-dimensional printing with growth factors.J Control Release. 2019 Feb 10;295:50-59. doi: 10.1016/j.jconrel.2018.12.035. Epub 2018 Dec 20. J Control Release. 2019. PMID: 30579982 Free PMC article. Review.
-
Localized SDF-1α Delivery Increases Pro-Healing Bone Marrow-Derived Cells in the Supraspinatus Muscle Following Severe Rotator Cuff Injury.Regen Eng Transl Med. 2018 Jun;4(2):92-103. doi: 10.1007/s40883-018-0052-4. Epub 2018 Apr 23. Regen Eng Transl Med. 2018. PMID: 30288396 Free PMC article.
-
Tooth-Supporting Hard Tissue Regeneration Using Biopolymeric Material Fabrication Strategies.Molecules. 2020 Oct 19;25(20):4802. doi: 10.3390/molecules25204802. Molecules. 2020. PMID: 33086674 Free PMC article. Review.
-
Technological Approaches for Improving Vaccination Compliance and Coverage.Vaccines (Basel). 2020 Jun 16;8(2):304. doi: 10.3390/vaccines8020304. Vaccines (Basel). 2020. PMID: 32560088 Free PMC article. Review.
-
[Research progress on controlled release of various growth factors in bone regeneration].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019 Jun 15;33(6):750-755. doi: 10.7507/1002-1892.201901116. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2019. PMID: 31198005 Free PMC article. Review. Chinese.
References
-
- Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012;3:1269. - PubMed
-
- Lindahl U, Li J. Int. Rev. Cell Mol. Biol. 1st. Elsevier Inc; 2009. Chapter 3 Interactions Between Heparan Sulfate and Proteins—Design and Functional Implications; pp. 105–159. - PubMed
-
- Lee KY, Yuk SH. Polymeric protein delivery systems. Prog. Polym. Sci. 2007;32:669–697.
-
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009;8:457–470. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

