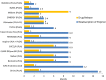
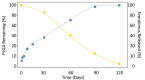
Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents
- PMID: 28017123
- PMCID: PMC5452149
- DOI: 10.2174/1573403X12666161222155230
Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents
Abstract
Drug-eluting stents (DES) have been shown to significantly reduce clinical and angiographic restenosis compared to bare metal stents (BMS). The polymer coatings on DES elute antiproliferative drugs to inhibit intimal proliferation and prevent restenosis after stent implantation. Permanent polymers which do not degrade in vivo may increase the likelihood of stent-related delayed arterial healing or polymer hypersensitivity. In turn, these limitations may contribute to an increased risk of late clinical events. Intuitively, a polymer which degrades after completion of drug release, leaving an inert metal scaffold in place, may improve arterial healing by removing a chronic source of inflammation, neoatherosclerosis, and/or late thrombosis. In this way, a biodegradable polymer may reduce late ischemic events. Additionally, improved healing after stent implantation could reduce the requirement for long-term dual antiplatelet therapy and the associated risk of bleeding and cost. This review will focus on bioabsorbable polymer-coated DES currently being evaluated in clinical trials.
Keywords: BMS; Bioabsorbable polymer; abluminal coating; clinical trials; drug-eluting stent..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
References
-
- Serruys P.W., de Jaegere P., Kiemeneij F., et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 1994;331(8):489–495. - PubMed
-
- Goldstein L.B., Bushnell C.D., Adams R.J., et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. - PubMed
-
- Indolfi C., De Rosa S., Colombo A. Bioresorbable vascular scaffolds — basic concepts and clinical outcome. Nat. Rev. Cardiol. 2016;13:719–729. - PubMed
-
- Ramcharitar S., Vaina S., Serruys P.W. The next generation of drug-eluting stents: what’s on the horizon? Am J Cardiovasc Drugs Drugs Devices Interv. 2007;7(2):81–93. - PubMed
-
- Busch R., Strohbach A., Peterson S., Sternberg K., Felix S. Parameters of endothelial function are dependent on polymeric surface material. Biomed. Tech. (Berl.) 2013 [Epub ahead of print]. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources