Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer
- PMID: 28017306
- PMCID: PMC7523385
- DOI: 10.1016/j.ygyno.2016.12.012
Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer
Abstract
Objective: To examine trends of adjuvant radiotherapy choice and to examine associations between pelvic lymphadenectomy and radiotherapy choice for women with early-stage endometrial cancer.
Methods: The Surveillance, Epidemiology, and End Results Program was used to identify surgically treated stage I-II endometrial cancer between 1983 and 2012 (type 1 n=79,474, and type 2 n=25,020). Piecewise linear regression models were used to examine temporal trends of intracavitary brachytherapy (ICBT) and whole pelvic radiotherapy (WPRT) use, pelvic lymphadenectomy rate, and sampled node counts. Multivariable binary logistic regression models were used to identify independent predictors for ICBT use.
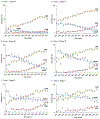
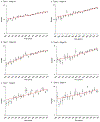
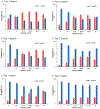
Results: There was a significant increase in ICBT use and decrease in WPRT use during the study period. ICBT use exceeded WPRT use in 2003 for type 1 stage IA, and in 2007 for type 1 stage IB and type 2 stage IA diseases. In addition, number of sampled pelvic nodes significantly increased over time in type 1-2 stage I-II diseases (mean, 7.0-12.7 in 1988 to 15.2-17.6 in 2012, all P<0.001). On multivariable analysis, extent of sampled pelvic nodes was significantly associated with ICBT use for type 1 cancer: adjusted-odds ratios for 1-10 and >10 nodes versus no lymphadenectomy in stage IA (1.38/2.40), IB (2.75/6.32), and II (1.36/2.91) diseases. Similar trends were observed for type 2 cancer: adjusted-odds ratios for stage IA (1.69/3.73), IB (2.25/5.65), and II (1.36/2.19) diseases.
Conclusion: Our results suggest that surgeons and radiation oncologists are evaluating the extent of pelvic lymphadenectomy when counseling women with early-stage endometrial cancer for adjuvant radiotherapy.
Keywords: Adjuvant radiotherapy; Early stage; Endometrial cancer; Intracavitary brachytherapy; Whole pelvic radiotherapy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
There is no conflict of interest in all authors.
Figures

References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA Cancer J. Clin 66 (2016) 7–30. - PubMed
-
- Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ, Contemporary management of endometrial cancer, Lancet 379 (2012) 1352–1360. - PubMed
-
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG, A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study, Gynecol. Oncol 92 (2004) 744–751. - PubMed
-
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, De Winter KA, Lutgens LC, van den Bergh AC, van de Steen-Banasik E, Beerman H, van Lent M, Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. Post operative radiation therapy in endometrial carcinoma, Lancet 355 (2000) 1404–1411. - PubMed
-
- Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Kroese MC, van Bunningen BN, Ansink AC, van Putten WL, Creutzberg CL, Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial, Lancet 375 (2010)816–823. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

