Mutations in EBF3 Disturb Transcriptional Profiles and Cause Intellectual Disability, Ataxia, and Facial Dysmorphism
- PMID: 28017373
- PMCID: PMC5223027
- DOI: 10.1016/j.ajhg.2016.11.012
Mutations in EBF3 Disturb Transcriptional Profiles and Cause Intellectual Disability, Ataxia, and Facial Dysmorphism
Abstract
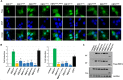
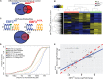
From a GeneMatcher-enabled international collaboration, we identified ten individuals affected by intellectual disability, speech delay, ataxia, and facial dysmorphism and carrying a deleterious EBF3 variant detected by whole-exome sequencing. One 9-bp duplication and one splice-site, five missense, and two nonsense variants in EBF3 were found; the mutations occurred de novo in eight individuals, and the missense variant c.625C>T (p.Arg209Trp) was inherited by two affected siblings from their healthy mother, who is mosaic. EBF3 belongs to the early B cell factor family (also known as Olf, COE, or O/E) and is a transcription factor involved in neuronal differentiation and maturation. Structural assessment predicted that the five amino acid substitutions have damaging effects on DNA binding of EBF3. Transient expression of EBF3 mutant proteins in HEK293T cells revealed mislocalization of all but one mutant in the cytoplasm, as well as nuclear localization. By transactivation assays, all EBF3 mutants showed significantly reduced or no ability to activate transcription of the reporter gene CDKN1A, and in situ subcellular fractionation experiments demonstrated that EBF3 mutant proteins were less tightly associated with chromatin. Finally, in RNA-seq and ChIP-seq experiments, EBF3 acted as a transcriptional regulator, and mutant EBF3 had reduced genome-wide DNA binding and gene-regulatory activity. Our findings demonstrate that variants disrupting EBF3-mediated transcriptional regulation cause intellectual disability and developmental delay and are present in ∼0.1% of individuals with unexplained neurodevelopmental disorders.
Keywords: EBF3; de novo mutation; developmental delay; gene regulation; intellectual disability; transcription factor.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. - PubMed
-
- Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. - PubMed
-
- Kortüm F., Caputo V., Bauer C.K., Stella L., Ciolfi A., Alawi M., Bocchinfuso G., Flex E., Paolacci S., Dentici M.L. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat. Genet. 2015;47:661–667. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

