Biallelic Mutations in MYPN, Encoding Myopalladin, Are Associated with Childhood-Onset, Slowly Progressive Nemaline Myopathy
- PMID: 28017374
- PMCID: PMC5223057
- DOI: 10.1016/j.ajhg.2016.11.017
Biallelic Mutations in MYPN, Encoding Myopalladin, Are Associated with Childhood-Onset, Slowly Progressive Nemaline Myopathy
Abstract
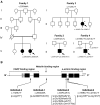
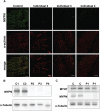
Nemaline myopathy (NM) is a common form of congenital nondystrophic skeletal muscle disease characterized by muscular weakness of proximal dominance, hypotonia, and respiratory insufficiency but typically not cardiac dysfunction. Wide variation in severity has been reported. Intranuclear rod myopathy is a subtype of NM in which rod-like bodies are seen in the nucleus, and it often manifests as a severe phenotype. Although ten mutant genes are currently known to be associated with NM, only ACTA1 is associated with intranuclear rod myopathy. In addition, the genetic cause remains unclear in approximately 25%-30% of individuals with NM. We performed whole-exome sequencing on individuals with histologically confirmed but genetically unsolved NM. Our study included individuals with milder, later-onset NM and identified biallelic loss-of-function mutations in myopalladin (MYPN) in four families. Encoded MYPN is a sarcomeric protein exclusively localized in striated muscle in humans. Individuals with identified MYPN mutations in all four of these families have relatively mild, childhood- to adult-onset NM with slowly progressive muscle weakness. Walking difficulties were recognized around their forties. Decreased respiratory function, cardiac involvement, and intranuclear rods in biopsied muscle were observed in two individuals. MYPN was localized at the Z-line in control skeletal muscles but was absent from affected individuals. Homozygous knockin mice with a nonsense mutation in Mypn showed Z-streaming and nemaline-like bodies adjacent to a disorganized Z-line on electron microscopy, recapitulating the disease. Our results suggest that MYPN screening should be considered in individuals with mild NM, especially when cardiac problems or intranuclear rods are present.
Keywords: MYPN; congenital myopathy; intranuclear rod myopathy; nemaline myopathy; whole-exome sequencing.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Romero N.B., Sandaradura S.A., Clarke N.F. Recent advances in nemaline myopathy. Curr. Opin. Neurol. 2013;26:519–526. - PubMed
-
- Nowak K.J., Wattanasirichaigoon D., Goebel H.H., Wilce M., Pelin K., Donner K., Jacob R.L., Hübner C., Oexle K., Anderson J.R. Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat. Genet. 1999;23:208–212. - PubMed
-
- Laing N.G., Wilton S.D., Akkari P.A., Dorosz S., Boundy K., Kneebone C., Blumbergs P., White S., Watkins H., Love D.R. A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy NEM1. Nat. Genet. 1995;10:249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

