Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!)
- PMID: 28017403
- PMCID: PMC5364328
- DOI: 10.1016/S0140-6736(16)32621-6
Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!)
Erratum in
-
Department of Error.Lancet. 2017 Feb 4;389(10068):504. doi: 10.1016/S0140-6736(16)32633-2. Epub 2016 Dec 24. Lancet. 2017. PMID: 28024962 Free PMC article. No abstract available.
-
Department of Error.Lancet. 2017 Feb 4;389(10068):504. doi: 10.1016/S0140-6736(17)30210-6. Lancet. 2017. PMID: 28170333 Free PMC article. No abstract available.
Abstract
Background: rVSV-ZEBOV is a recombinant, replication competent vesicular stomatitis virus-based candidate vaccine expressing a surface glycoprotein of Zaire Ebolavirus. We tested the effect of rVSV-ZEBOV in preventing Ebola virus disease in contacts and contacts of contacts of recently confirmed cases in Guinea, west Africa.
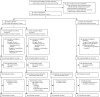
Methods: We did an open-label, cluster-randomised ring vaccination trial (Ebola ça Suffit!) in the communities of Conakry and eight surrounding prefectures in the Basse-Guinée region of Guinea, and in Tomkolili and Bombali in Sierra Leone. We assessed the efficacy of a single intramuscular dose of rVSV-ZEBOV (2×107 plaque-forming units administered in the deltoid muscle) in the prevention of laboratory confirmed Ebola virus disease. After confirmation of a case of Ebola virus disease, we definitively enumerated on a list a ring (cluster) of all their contacts and contacts of contacts including named contacts and contacts of contacts who were absent at the time of the trial team visit. The list was archived, then we randomly assigned clusters (1:1) to either immediate vaccination or delayed vaccination (21 days later) of all eligible individuals (eg, those aged ≥18 years and not pregnant, breastfeeding, or severely ill). An independent statistician generated the assignment sequence using block randomisation with randomly varying blocks, stratified by location (urban vs rural) and size of rings (≤20 individuals vs >20 individuals). Ebola response teams and laboratory workers were unaware of assignments. After a recommendation by an independent data and safety monitoring board, randomisation was stopped and immediate vaccination was also offered to children aged 6-17 years and all identified rings. The prespecified primary outcome was a laboratory confirmed case of Ebola virus disease with onset 10 days or more from randomisation. The primary analysis compared the incidence of Ebola virus disease in eligible and vaccinated individuals assigned to immediate vaccination versus eligible contacts and contacts of contacts assigned to delayed vaccination. This trial is registered with the Pan African Clinical Trials Registry, number PACTR201503001057193.
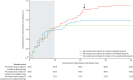
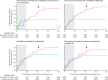
Findings: In the randomised part of the trial we identified 4539 contacts and contacts of contacts in 51 clusters randomly assigned to immediate vaccination (of whom 3232 were eligible, 2151 consented, and 2119 were immediately vaccinated) and 4557 contacts and contacts of contacts in 47 clusters randomly assigned to delayed vaccination (of whom 3096 were eligible, 2539 consented, and 2041 were vaccinated 21 days after randomisation). No cases of Ebola virus disease occurred 10 days or more after randomisation among randomly assigned contacts and contacts of contacts vaccinated in immediate clusters versus 16 cases (7 clusters affected) among all eligible individuals in delayed clusters. Vaccine efficacy was 100% (95% CI 68·9-100·0, p=0·0045), and the calculated intraclass correlation coefficient was 0·035. Additionally, we defined 19 non-randomised clusters in which we enumerated 2745 contacts and contacts of contacts, 2006 of whom were eligible and 1677 were immediately vaccinated, including 194 children. The evidence from all 117 clusters showed that no cases of Ebola virus disease occurred 10 days or more after randomisation among all immediately vaccinated contacts and contacts of contacts versus 23 cases (11 clusters affected) among all eligible contacts and contacts of contacts in delayed plus all eligible contacts and contacts of contacts never vaccinated in immediate clusters. The estimated vaccine efficacy here was 100% (95% CI 79·3-100·0, p=0·0033). 52% of contacts and contacts of contacts assigned to immediate vaccination and in non-randomised clusters received the vaccine immediately; vaccination protected both vaccinated and unvaccinated people in those clusters. 5837 individuals in total received the vaccine (5643 adults and 194 children), and all vaccinees were followed up for 84 days. 3149 (53·9%) of 5837 individuals reported at least one adverse event in the 14 days after vaccination; these were typically mild (87·5% of all 7211 adverse events). Headache (1832 [25·4%]), fatigue (1361 [18·9%]), and muscle pain (942 [13·1%]) were the most commonly reported adverse events in this period across all age groups. 80 serious adverse events were identified, of which two were judged to be related to vaccination (one febrile reaction and one anaphylaxis) and one possibly related (influenza-like illness); all three recovered without sequelae.
Interpretation: The results add weight to the interim assessment that rVSV-ZEBOV offers substantial protection against Ebola virus disease, with no cases among vaccinated individuals from day 10 after vaccination in both randomised and non-randomised clusters.
Funding: WHO, UK Wellcome Trust, the UK Government through the Department of International Development, Médecins Sans Frontières, Norwegian Ministry of Foreign Affairs (through the Research Council of Norway's GLOBVAC programme), and the Canadian Government (through the Public Health Agency of Canada, Canadian Institutes of Health Research, International Development Research Centre and Department of Foreign Affairs, Trade and Development).
Copyright © 2017 World Health Organization. Published by Elsevier Ltd/Inc/BV. All rights reserved. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
First Ebola virus vaccine to protect human beings?Lancet. 2017 Feb 4;389(10068):479-480. doi: 10.1016/S0140-6736(16)32618-6. Epub 2016 Dec 23. Lancet. 2017. PMID: 28017402 No abstract available.
-
Questionable efficacy of the rVSV-ZEBOV Ebola vaccine - Authors' reply.Lancet. 2018 Mar 17;391(10125):1021-1022. doi: 10.1016/S0140-6736(18)30559-2. Epub 2018 Mar 15. Lancet. 2018. PMID: 29565012 No abstract available.
-
Questionable efficacy of the rVSV-ZEBOV Ebola vaccine.Lancet. 2018 Mar 17;391(10125):1021. doi: 10.1016/S0140-6736(18)30560-9. Epub 2018 Mar 15. Lancet. 2018. PMID: 29565013 No abstract available.
References
-
- WHO Ebola virus disease fact sheet. 2016. http://www.who.int/mediacentre/factsheets/fs103/en/ (accessed Nov 30, 2016).
-
- Ministry of Health Guinea Ebola situation, report 655. Jan 30, 2016. http://guinea-ebov.github.io/code/files/sitreps/GUINEA_EBOLA_SITREP%20N%... (accessed Nov 30, 2016).
-
- Kanapathipillai R, Henao Restrepo AM, Fast P. Ebola vaccine—an urgent international priority. N Engl J Med. 2014;371:2249–2251. - PubMed
-
- Jones SM, Stroher U, Fernando L. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196(suppl 2):S404–S412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

