Ginsenoside Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression
- PMID: 28017961
- PMCID: PMC5309758
- DOI: 10.1038/aps.2016.135
Ginsenoside Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression
Abstract
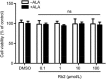
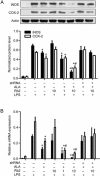
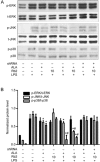
Recent studies confirm that chronic low-grade inflammation is closely associated with metabolic syndromes, and anti-inflammatory therapy is a potential approach for treating cardiovascular diseases and type 2 diabetes. Accumulating evidence suggests that GPR120 activation is a feasible solution to ameliorating chronic inflammation and improving glucose metabolism. In this study we investigated whether ginsenoside Rb2 (Rb2), which exhibited regulatory activities in glucose and lipid metabolism, affected GPR120 expression in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells, and examined the contribution of GPR120 activation to reducing the LPS-induced inflammatory response. LPS (100 ng/mL) activated the macrophages, resulting in dramatic increases in TNF-α, IL-6, IL-1β and NO production. Treatment with a ω-3 fatty acid α-linolenic acid (ALA, 50 μmol/L) produced moderate reduction in LPS-stimulated inflammatory cytokines and NO production (TNF-α and IL-6 were decreased by 46% and 42%, respectively). Pre-incubation with Rb2 (1 or 10 μmol/L) for 12 h before ALA treatment dramatically amplified the inhibitory effects of ALA (TNF-α and IL-6 were decreased by 74% and 86%, respectively). Compared to the treatment with ALA alone, pre-incubation with Rb2 resulted in a more prominent reduction in LPS-stimulated expression of iNOS and COX-2 and LPS-stimulated IKK/NF-κB phosphorylation and MAPK pathway activation. Rb2 (0.1-100 μmol/L) dose- and time-dependently increased both mRNA and protein expression of GPR120 in RAW264.7 cells, but treatment with Rb2 alone did not exert anti-inflammatory effect in LPS-activated RAW264.7 cells. In RAW264.7 cells transfected with GPR120 shRNA, the ameliorating effects of Rb2 on LPS-induced inflammation were abolished. In conclusion, Rb2 exerts anti-inflammatory effect in LPS-stimulated mouse macrophage RAW264.7 cells in vitro by increasing GPR120 expression and subsequently enhancing ω-3 fatty acid-induced GPR120 activation.
Figures



Similar articles
-
Amelioration of an LPS-induced inflammatory response using a methanolic extract of Lagerstroemia ovalifolia to suppress the activation of NF-κB in RAW264.7 macrophages.Int J Mol Med. 2016 Aug;38(2):482-90. doi: 10.3892/ijmm.2016.2646. Epub 2016 Jun 16. Int J Mol Med. 2016. PMID: 27314211
-
The p38 MAPK inhibitor JLU1124 inhibits the inflammatory response induced by lipopolysaccharide through the MAPK-NF-κB pathway in RAW264.7 macrophages.Int Immunopharmacol. 2013 Nov;17(3):785-92. doi: 10.1016/j.intimp.2013.09.001. Epub 2013 Sep 23. Int Immunopharmacol. 2013. PMID: 24070708
-
Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages.J Agric Food Chem. 2012 Sep 26;60(38):9595-602. doi: 10.1021/jf301372g. Epub 2012 Sep 12. J Agric Food Chem. 2012. PMID: 22849695
-
The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity.Curr Opin Clin Nutr Metab Care. 2011 Jul;14(4):322-7. doi: 10.1097/MCO.0b013e3283479230. Curr Opin Clin Nutr Metab Care. 2011. PMID: 21587066 Review.
-
GPR120: Mechanism of action, role and potential for medical applications.Postepy Hig Med Dosw (Online). 2017 Nov 19;71(0):942-953. doi: 10.5604/01.3001.0010.5809. Postepy Hig Med Dosw (Online). 2017. PMID: 29176006 Review.
Cited by
-
Inhibitory role of ginsenoside Rb2 in endothelial senescence and inflammation mediated by microRNA‑216a.Mol Med Rep. 2021 Jun;23(6):415. doi: 10.3892/mmr.2021.12054. Epub 2021 Mar 31. Mol Med Rep. 2021. PMID: 33786633 Free PMC article.
-
Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system.Front Psychiatry. 2022 Aug 31;13:933704. doi: 10.3389/fpsyt.2022.933704. eCollection 2022. Front Psychiatry. 2022. PMID: 36117650 Free PMC article. Review.
-
Enhancing effect of Panax ginseng on Zip4-mediated zinc influx into the cytosol.J Ginseng Res. 2022 Mar;46(2):248-254. doi: 10.1016/j.jgr.2021.06.006. Epub 2021 Jun 15. J Ginseng Res. 2022. PMID: 35509828 Free PMC article.
-
Research Progress in Chinese Herbal Medicines for Treatment of Sepsis: Pharmacological Action, Phytochemistry, and Pharmacokinetics.Int J Mol Sci. 2021 Oct 14;22(20):11078. doi: 10.3390/ijms222011078. Int J Mol Sci. 2021. PMID: 34681737 Free PMC article. Review.
-
Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using 'Kunisato 35 Gou' Leaf Extract.Plants (Basel). 2023 Feb 19;12(4):948. doi: 10.3390/plants12040948. Plants (Basel). 2023. PMID: 36840296 Free PMC article.
References
-
- Dilshara MG, Jayasooriya RG, Kang CH, Lee S, Park SR, Jeong JW, et al. Downregulation of pro-inflammatory mediators by a water extract of Schisandra chinensis (Turcz) Baill fruit in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Environ Toxicol Pharmacol 2013; 36: 256–64. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35. - PubMed
-
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012; 18: 363–74. - PubMed
-
- Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 2016; 12: 15–28. - PubMed
-
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005; 11: 90–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

